Health Canada Ectd Sequence Description
Dr R W Middleton Herbal Medicines Regulatory Services Ltd. Primary healthcare encompasses a broad spectrum of care for preventative diagnostic and treatment services by clinicians who are generally responsible for co-ordinating the ongoing care of a patient.
The pilot for Clinical Trial regulatory activities in electronic Common Technical Document eCTD format concluded on August 31 2019.

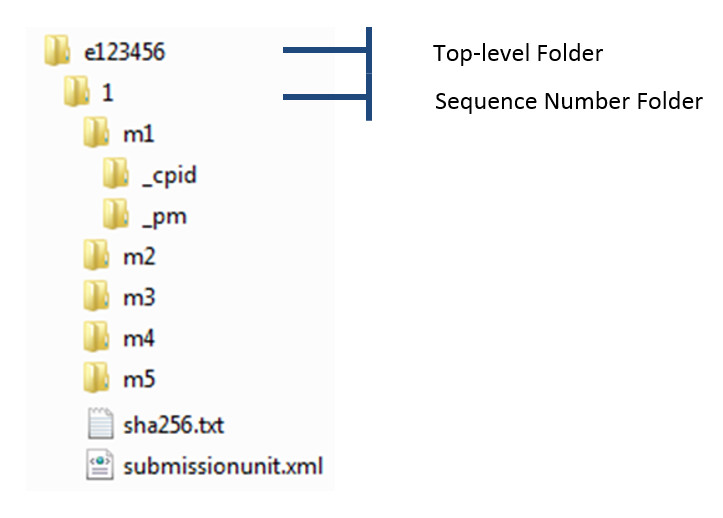
Health canada ectd sequence description. Notice - Canadian Module 1 Schema Version 22 extension of deadline 2013-02-11 Guidance for Industry. Every sequence received by Health Canada is validated a written eCTD Validation Report describing the deficiencies will be issued to the sponsor for regulatory transactions that. The figure shows how eCTD identifier e123456 links sequence number 0004 a subsequent regulatory activity for example a Supplement to a New Drug Submission.
Preparation of Drug Regulatory Activities in the Common Technical Document CTD Format. ECTD electronic Common Technical Document is a standard format of submitting Regulatory information such as applications supplements and reports to the concerned Health Authorities HAs. ECTD v40 Term Health Canada Term Description.
At this time Health Canada implementation is as per the following schedule. A collection of Submission Units or Regulatory Transactions make up the content for a Submission or Regulatory. Health Canada Implements eCTD for Clinical Trials Mar 3 2020 Health Canada the Canadian authority responsible for medical device regulation published a notice announcing the implementation of the electronic Common Technical Document eCTD format.
Notice - Validation rules for regulatory transactions submitted to Health Canada in the electronic Common Technical Document eCTD format 2019-02-05. Dd yyyy to Mmm. The Canadian Health Agency will be using the electronic Common Technical Document eCTD and non- electronic Common Technical Document non-eCTD validation rules version 50.
ECTD SUBMISSION SERVICES FROM ONIX. That means beginning the first day of 2018 Health Canada will accept submissions in electronic common technical document eCTD format only for certain regulatory filings which include. 11 Primary HealthCare in CanadaPrimary healthcare is a persons first point of contact with the healthcare system.
This document serves as the Technical Implementation Guide IG and a technical specification for the Health Canada eCTD v40 message using the Health Level 7 HL7 Regulated Product Submission RPS Release 2 Normative standard. The base message produced in eCTD v40. The health authority of Canadian landscape Health Canada HC has revised the submission requirements and mandated submissions to be in electronic format for certain filings post January 1st 2018.
The purpose of the. CTA submissions via eCTD is significant step in the propagation of eCTD. The structure of Modules 2 to 5 m2-m5 can be retrieved from the ICH Electronic Common Technical Document Specification Health Canadas Guidance Document.
Sequence description - For Period of Mmm. Sequence number 0013 a subsequent regulatory activity for example a Notifiable Change and sequence number 0019 a subsequent regulatory activity for example the forms summarizing Changes to Marketed Human New Drug Products. The util folder contains the technical auxiliary files for an eCTD the so-called DTD Stylesheets.
Each eCTD v40 message or Regulatory Transaction is considered a Submission Unit. Dd yyyy RMP-PV as a separate eCTD sequence using the corresponding sequence description - RMP version dated Mmm. 168 rows Sponsors are encouraged to use a commercially available tool to validate their regulatory.
Notice Mandatory use of the Electronic Common Technical Document eCTD format 2019-02-05. Notices of Change Level III to sequence number 0000 the original regulatory activity New Drug. As of March 31st 2013 Health Canada will no longer be accepting regulatory activities.
As of September 30th 2012 Health Canada will be accepting regulatory activities built using the revised Canadian Module 1 Schema Version 22. Dd yyyy UD-PV as a separate eCTD sequence using the corresponding sequence description - eg Response to MHPD Requests dated Mmm. Health Canada accepts electronic Common Technical Document eCTD submissions for pre-clinical trial application consultation meetings clinical trial applications CTA amendments notifications and responses for post-clearance data related to those applications.
It provides a harmonized solution to implement the Common Technical Document CTD electronically. Health Canada is pleased to announce that the pilot was successful. From 1 November 2021 the Therapeutic Goods Administration TGA will begin a staged transition to Electronic Common Technical Document-only eCTD-only for all prescription medicines.
Electronic Submissions or eSubmissions electronic Submissions or eSubmissions are the electronic presentation of information submitted to the TGA or other. Therefore implementation of Clinical Trials regulatory activities in eCTD format will begin immediately for the following. Health Canada is the regional authority for Canada and thus sets the Canadian.
Http Www Aipm Org Netcat Files Userfiles Aipm Files For Download Practical Experience Proposal For Ectd Implementation Pdf

Guidance For Industry On Providing Regulatory Information In Ectd Fo

Resource Library Case Studies Authorization Approval Pharmalex

Guidance Document Creation Of The Canadian Module 1 Backbone
Draft Guidance Document Profile Canadian Module 1 Technical Implementation Guide For The Electronic Common Technical Document Ectd V4 0 Format Canada Ca

Ectdtemplates Extedo S Ectd Word Templates For Fda Ema Health Canada Eaeu And Asia Youtube

Caring For Your Teeth And Mouth Children Health Canada Oral Health Tooth Chart Kids Health

Tooth Eruption Chart Tooth Chart Teeth Eruption Dental Teeth

Key Factors For In Licensing An Ectd Dossier Find Out More

Health Canada Implements Ectd For Clinical Trials Regdesk

Health Canada Guidance For Biotechnology Products Professor Peiva

E Learning Ectd Preparation And Submission Youtube
Ca Health Canada Ectd Compiler Ectd Office Ectd Nees Vnees Publishing Software Solution
Http Publications Gc Ca Collections Collection 2021 Sc Hc H164 293 2019 Eng Pdf

Health Canada Implements Ectd For Clinical Trials Regdesk

Ich S Ectd Version 4 0 Objectives Major Updates Resulting Advantages And Possible Challenges Techsol Corporation

Ectdtemplates Extedo S Ectd Word Templates For Fda Ema Health Canada Eaeu And Asia Youtube

Electronic Common Technical Document Limswiki

Posting Komentar untuk "Health Canada Ectd Sequence Description"