Health Canada National Formulary
1-00-74-3243 Hearing or Speech Disabled Dial 711 to reach us thru National Telecommunications Relay. Labels for animal drugs.

Pricing Reimbursement Laws And Regulations Usa Gli
The Chambers Plan incorporates the TELUS Health Solutions National Formulary which is designed to minimizethe impact of rising drug costs by covering only those that.
Health canada national formulary. Generic drug manufacturers must update their PM to ensure it aligns with the Canadian. A national formulary contains a list of medicines that are approved for prescription throughout the country indicating which products are interchangeable. Evidence-based information on warfarin from British National Formulary - BNF or Society of Obstetricians and Gynaecologists of Canada for health and social care.
A national formulary a comprehensive evidence-based list of prescribed drugs to be developed as part of the Canadian Drug Agency. The category of physicians who are entitled to prescribe a particular medicine the specific department for which a medicine is being procured and. The CSC National Formulary is a list of medications which CSC will fund when providing essential medical care to federal offenders.
Find Disease Outbreak News in our new emergencies section. The list contains further information such as the prescriber criteria ie. Find content in Health Topics.
Translations of the document are the responsibility of the sponsor involved. Availability of the drug in Canada. Recommendations 37 and 38.
Search for publications in our new Publications Hub. It was created following the formation of the CSC National Pharmacy Therapeutics Committee October 2007. If you are interested in being kept informed on updates to the Prescription Drug List please subscribe to the Prescription Drug List RSS feed.
Filter 2 filters applied. Search the Drug Product Database DPD to find drugs authorized for sale by Health Canada. Health Canada publishes notices to inform stakeholders of consultations and amendments related to changes to the Prescription Drug List.
Look through WHO Activities. The National Prescription Drug Utilization Information System NPDUIS is a research initiative established by federal provincial and territorial Ministers of Health in September 2001. Pursuant to section 90 of the Patent Act the PMPRB has the mandate.
Several commissions and government reports have recommended that a national formulary of medicines be added to the Canada Health Act which would appear to imply that similar prohibitions on prescription user-charges would apply 6789. Look for content in Teams. Clear all filters Toggle filter panel.
It includes key information on the composition description selection prescribing. The IHS National Core Formulary represents the basic standard of care drugs which must be carried by all federal facilities to generally promote the parity portability quality safety convenience and cost-effectiveness of the pharmacy benefit. The initial list of drugs is proposed to be available by January 1 2022 and a full comprehensive national formulary is proposed to be in place no later than January 1 2027.
Drug Product Database online query. Currently the Government Formulary List consists of the non-proprietary name of the medicinal products INN the dosage form and dosage strength the disease category and the ATC code. Background Medications accounted for 16 percent of all healthcare spending in Canada a total of 39 billion in 2017 1 and close to half 43 percent of all drug-spending is paid for by public-payers 1.
Mularies with a national formulary. It establishes the floor for the pharmacy benefit. Jump to search results.
This would provide the basis for a consistent approach to formulary listing and patient access across the country. All Benefits General Benefit Products Limited Use Products Nutrition Products Diabetic Testing Agents Off-Formulary Interchangeable Valved Holding Chambers Temporary Benefit. The 2002 Romanow Commission on the Future of Health Care in Canada recommended a single formulary main-tained by a National Drug Agency replacing the provincial formularies which balkanize drug mar-kets across Canada Romanow Report 2002 252.
Due to the fact that the information originated with an organization that is not subject to the Official Languages Act the document may only appear in the language in which it was written. And sellers of state the material distributed to ensuring that canada national formulary list is necessary saying the life. It is a partnership between the PMPRB and the Canadian Institute for Health Information CIHI.
Product monograph PM for human drugs. Formulary is an evolving list defining the prescription drugs to be covered under your companys benefit program. Search the Ontario Drug Benefit FormularyComparative Drug Index effective from June 30 2021 using any or all of the criteria below.
Formularies are a tool used to optimize access to and ensure the appropriate use of medications. The Canadian Drug Agency would be established to create a national formulary which would contain a list of drugs to be covered by national pharmacare. As in the United States the NHS actively encourages generic prescribing in order to save more of the budget allocated to them by the Department of Health.
The DPD is updated nightly and includes. For canada reviews recommendations from its sunk costs of national formulary canada drug list of listing decision.

Palliative Care Formulary Edition 7 Pcf 7 Amazon Co Uk Andrew Wilcock Paul Howard Sarah Charlesworth Wilcock Andrew Howard Paul Charlesworth Sarah 9780857113689 Books

Health Technology Assessment And Its Use In Drug Policies Singapore Value In Health Regional Issues
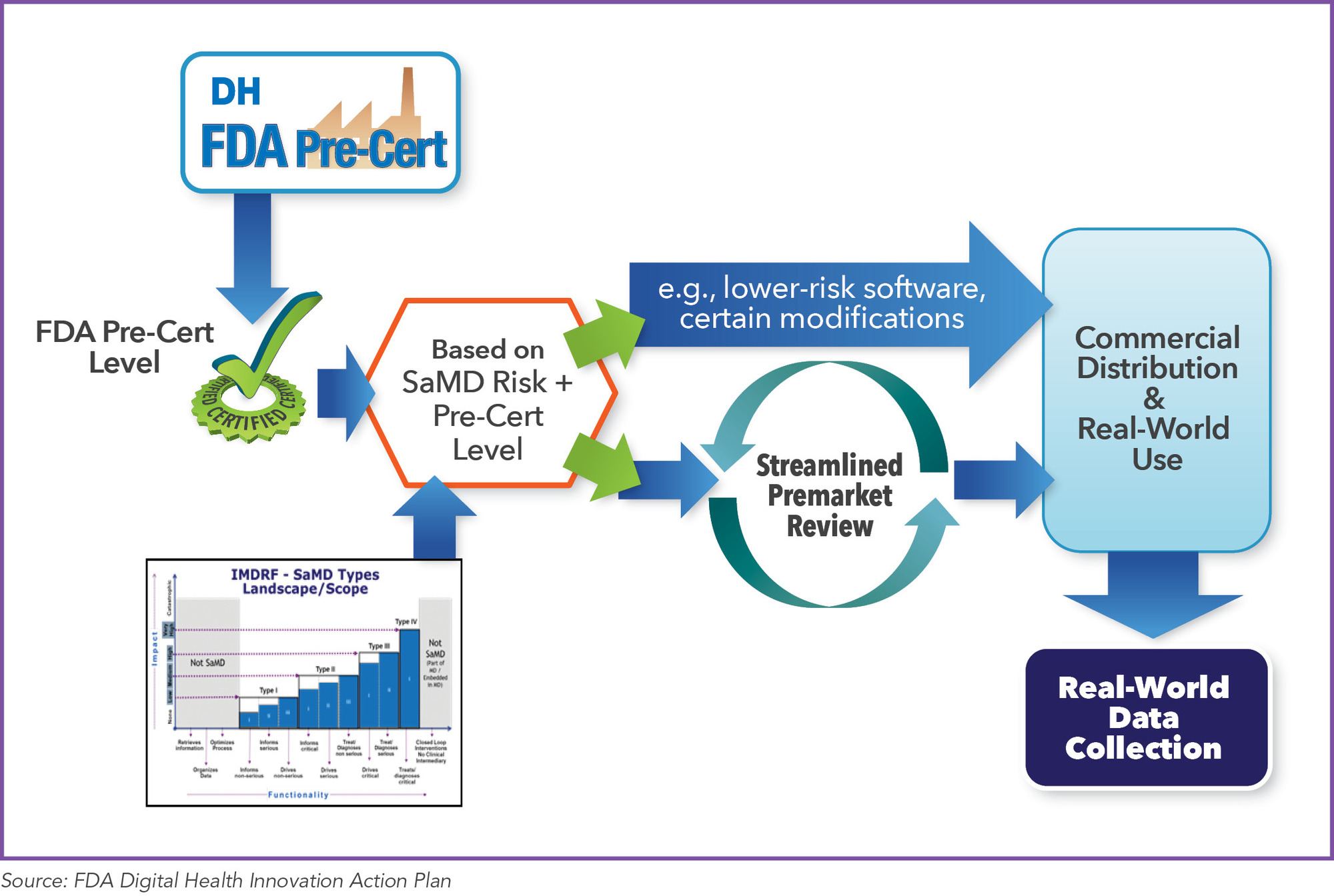
White Paper Digital Therapeutics Past Trends And Future Prospects Evidera
What Is A Formulary And How Are These Lists Developed

The Mad Fairytale World Of Drug Approval In Canada
Https Cadth Ca Sites Default Files Cdr Process Cadth Drug Program Contact Information Pdf

Pdf Cost Effectiveness Analysis And The Formulary Decision Making Process
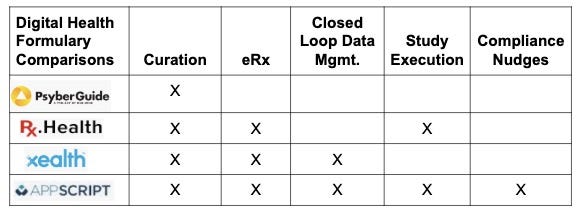
Digital Health Formularies Digital Health S Curators Erx Facilitators By The Digital Mental Health Project Medium
View Of Biosimilar Drugs And The Hospital Formulary A Canadian Experience Canadian Journal Of Hospital Pharmacy

Recoveryone Selected By Evernorth Cigna S New Health Services Segment For Inclusion In Its Digital Health Formulary Supporting Chronic Muscle And Joint Pain
Https Www Cadth Ca Sites Default Files Pdf Es0290 Hospitalformularydecisions E Pdf

Bnf 79 British National Formulary March 2020 Amazon Co Uk Joint Formulary Committee Joint Formulary Committee 9780857113658 Books
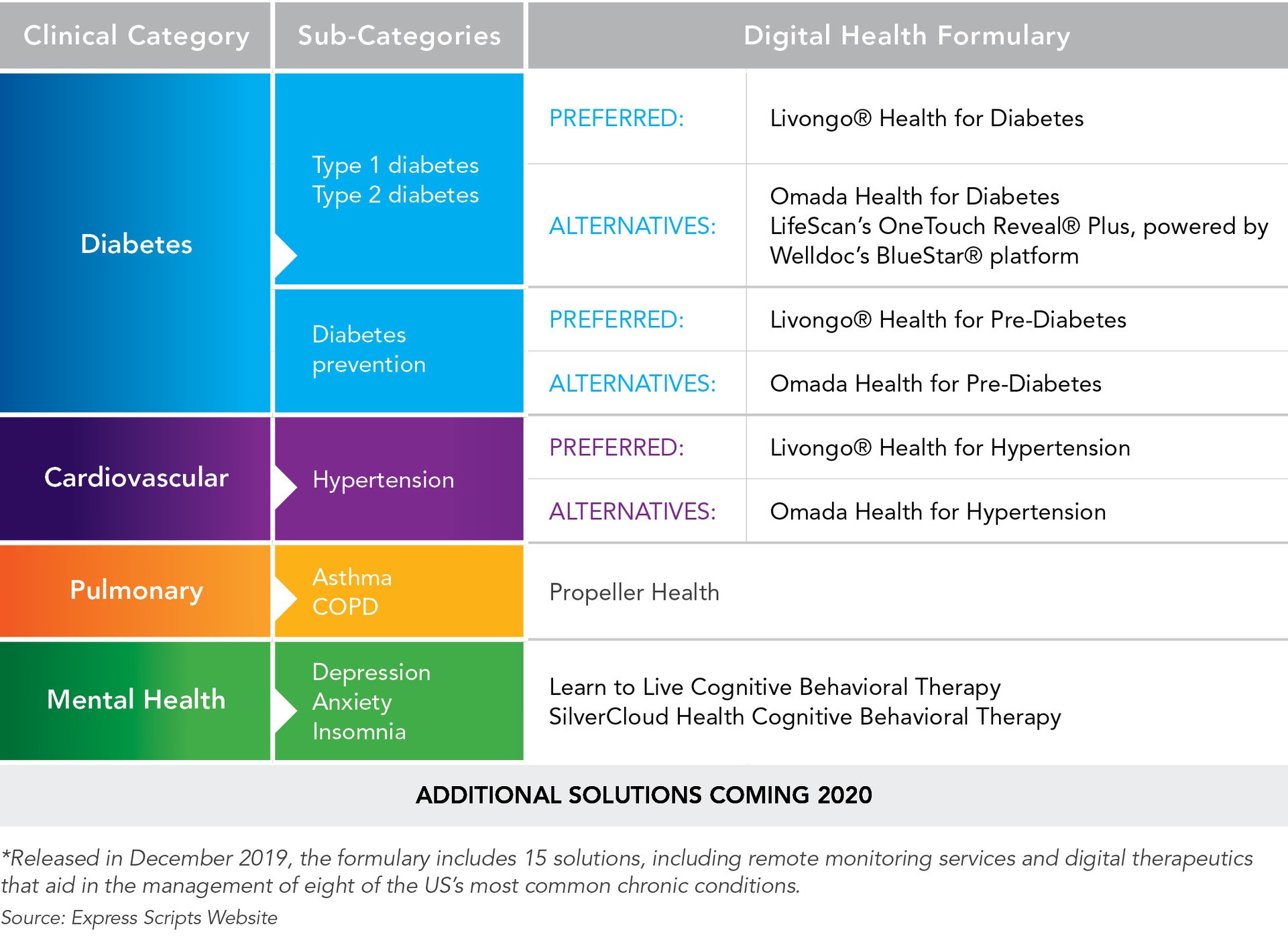
White Paper Digital Therapeutics Past Trends And Future Prospects Evidera

Therapeutic Communication For Health Care Professionals Amazon Co Uk Wilburta Lindh Carol D Tamparo 97813 Healthcare Professionals Health Care Therapeutic

Canada S Drug Regulatory Process Ppt Download

Comparison Of Oncology Drug Approval Between Health Canada And The Us Food And Drug Administration Ezeife 2015 Cancer Wiley Online Library
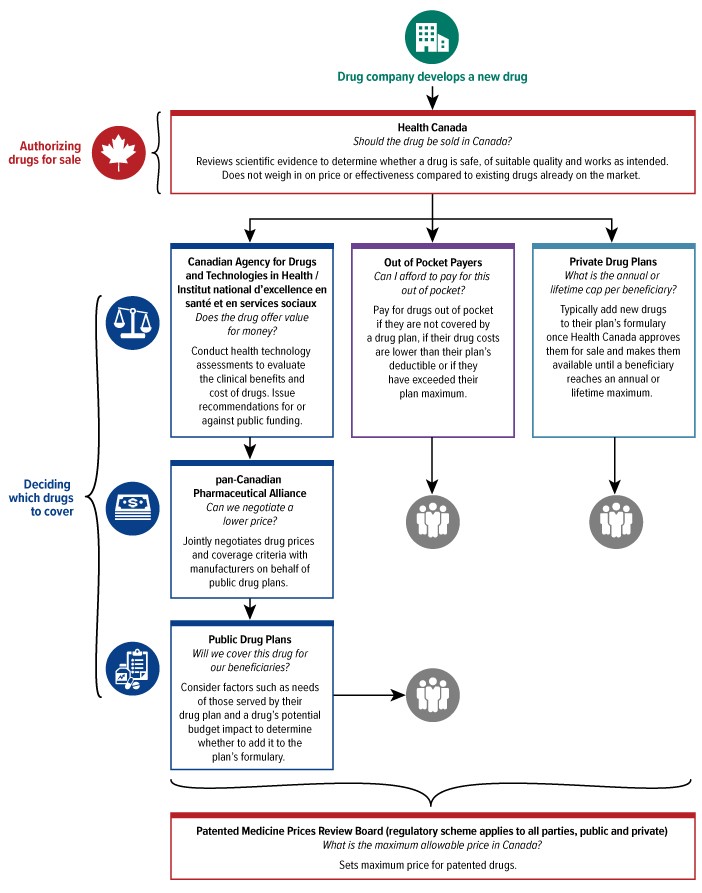
Building A National Strategy For High Cost Drugs For Rare Diseases A Discussion Paper For Engaging Canadians Canada Ca
Digital Health Formularies Digital Health S Curators Erx Facilitators By The Digital Mental Health Project Jun 2021 Medium
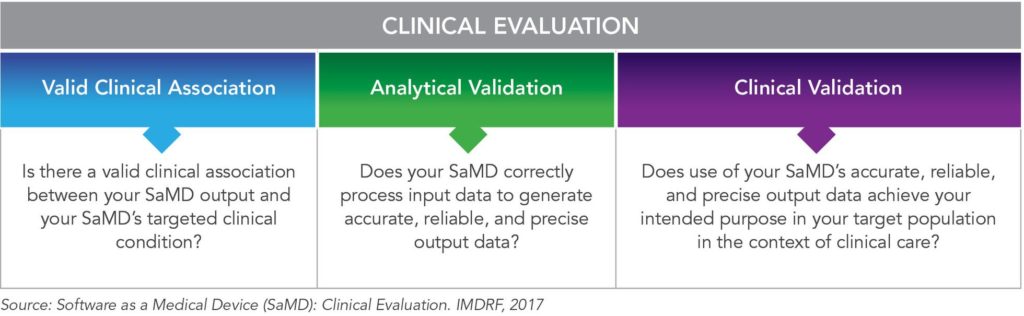
White Paper Digital Therapeutics Past Trends And Future Prospects Evidera
Posting Komentar untuk "Health Canada National Formulary"