Health Canada Medical Device Listing
Requirements for the development validation and routine control of a sterilization process for medical devices. Medical devices are categorized into four classes based on the level of risk associated with their use.

How To Stay Up To Date On The Ever Changing Landscape Of The Medical Electrical Device Regulatory World In Compliance Magazine
Health Canada Medical Device License MDL A Canadian Medical Device License MDL is required for companies selling Class II - IV medical devices in Canada.

Health canada medical device listing. Health Canada maintains and updates this list regularly. Thermometers and Class IV devices present the greatest potential risk eg. Manufacturers of medical devices classified as Class II III and IV must demonstrate compliance with ISO 13485 under MDSAP.
FOOD AND DRUGS ACT. Obtaining an MDL is comparable to the US FDA 510 k process. Class I medical devices masks surgical procedure or medical masks.
The list now includes new items in bold. Canada Health Canada Medical Devices Medical Device Active License Listing MDALL Medical Device Establishment Licence Listing MDEL Medical Device Incidents Recalls and Safety. The base URI for the Medical Devices Active Licence Listing is httpshealth-productscanadacaapimedical-devices and you can add parameters to it.
Any requests are made relative to this URI. The consultation period is open until July 6 2015. Selecting the Active Licence Search link takes you to the Medical Devices.
A primer on how Health Canada regulates medical devices. Health Canada currently requires compliance to the Medical Device Single Audit Program MDSAP which includes additional QMS procedures and regulatory requirements before they will approve your device for sale. 626 KB Regulations are current to 2021-06-16 and last amended on 2021-03-31.
Canadian regulators have updated their list of recognized standards pertaining to medical devices affecting manufacturers applying to Health Canada for Medical Device Licenses MDL. Dear visitor We have reorganized our Web site. Changes to the list include 15 new standards 11 new editions of current standards and removal of 14 standards.
The MDL is a product approval while a MDEL is a permit for the companydistributorimporter itself. The list was updated on May 20 2021 to include additional medical devices that require mandatory reporting by manufacturers and importers. Health Canada Code HC Class HC License 2632 Sterile Vinyl Exam Glove Pairs - Md 450Cs LYZ 1 80FMC 2 84209 2633 Sterile Vinyl Exam Glove Pairs Lg 450Cs LYZ 1 80FMC 2 84209 3145 Lap Sponge Sterile Pre-washed 12x 12- 405Cs GDY 1 79GEK 2.
Health Canada Proposes Changes to the List of Recognized Standards for Medical Devices Changes affecting the approval of medical devices are coming. This Application Programming Interface API allows developers to access that information in JSON and XML format for reuse in their own applications. 11 rows List of Medical Devices for Exceptional Importation and Sale Medical Device Medical.
Based on current performance reports available from Health Canada there has been some significant backlog build-up in applications at the Medical Devices Bureau MDB in Q1 and Q2 FY 201617 due to substantial capacity shortageEfforts were made by MDB to combat this trend which led to noticeable improvements in Q3. There are two types of licences issued by Health Canada for medical devices sold in Canada. Confirmation of device licence can be made by matching the product label to the company information device name and device identifier found in the MDALL database.
Levels 1 2 and 3 ATSM. HEalth canada Medical Devices Bureau MDB Performance. Canada QMS requirements for medical device companies.
An MDEL provides Health Canada assurance that medical devices sold or imported into Canada meet. A radiosurgery system that uses focused beams of radiation rather than a surgical knife to destroy tumours and other lesions. A newsletter on new and emerging health care technologies in Canada Medical device regulation in Canada.
Class I devices present the lowest potential risk eg. Sterilization of medical devices Information to be provided by the manufacturer for the processing of resterilizable medical devices ISO 17665-12006-Ed10 Sterilization of health care products Moist heat Part 1. Medical devices establishment licence listing.
A Medical Device Establishment Licence MDEL is a licence issued to Class I manufacturers as well as importers or distributors of all device classes to permit them to import or distribute a medical device in Canada. Medical Devices Active Licence Listing MDALL - Your reference tool for licensed medical devices in Canada. MDSAP includes compliance with the QMS requirements of the Canadian Medical Devices.
Shaded provisions are not in force. On May 6 2015 Health Canada released a draft List of Recognized Standards for medical devices for stakeholder consultation. See coming into force provision and notes where applicable.
Search the active Medical Devices Active Licence Listing MDALL to see if the product is a licensed Class II III or IV medical device.
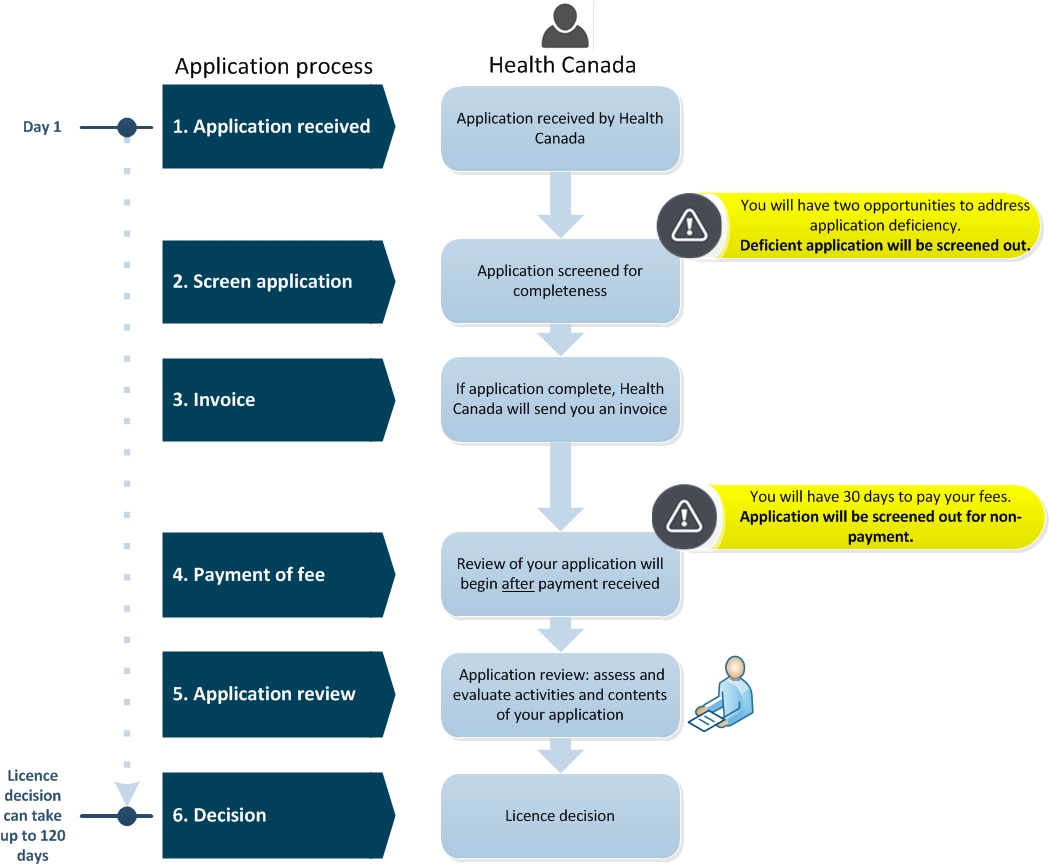
Frequently Asked Questions Medical Device Establishment Licensing And Fees Canada Ca

Spanish Medical Conversation Spanish Medical Translations Spanish Conversation Spanish Basics Learning Spanish

If A Customer Is Having An Allergic Reaction And Cannot Inject Their Epipen Themselves I Would Have To Know How To Do I Epipen Infographic Health And Wellness

Guidance On Medical Device Establishment Licensing Gui 0016 Canada Ca

2boxes 50pcs Each Box Disposable 3 Ply Non Woven Antiviral Facemasks Disposable Antiviral Face Mask

This Medical Invention Could Reduce Our Reliance On Stitches Stitches Medical Medical Stitches Medical Stitches Skin
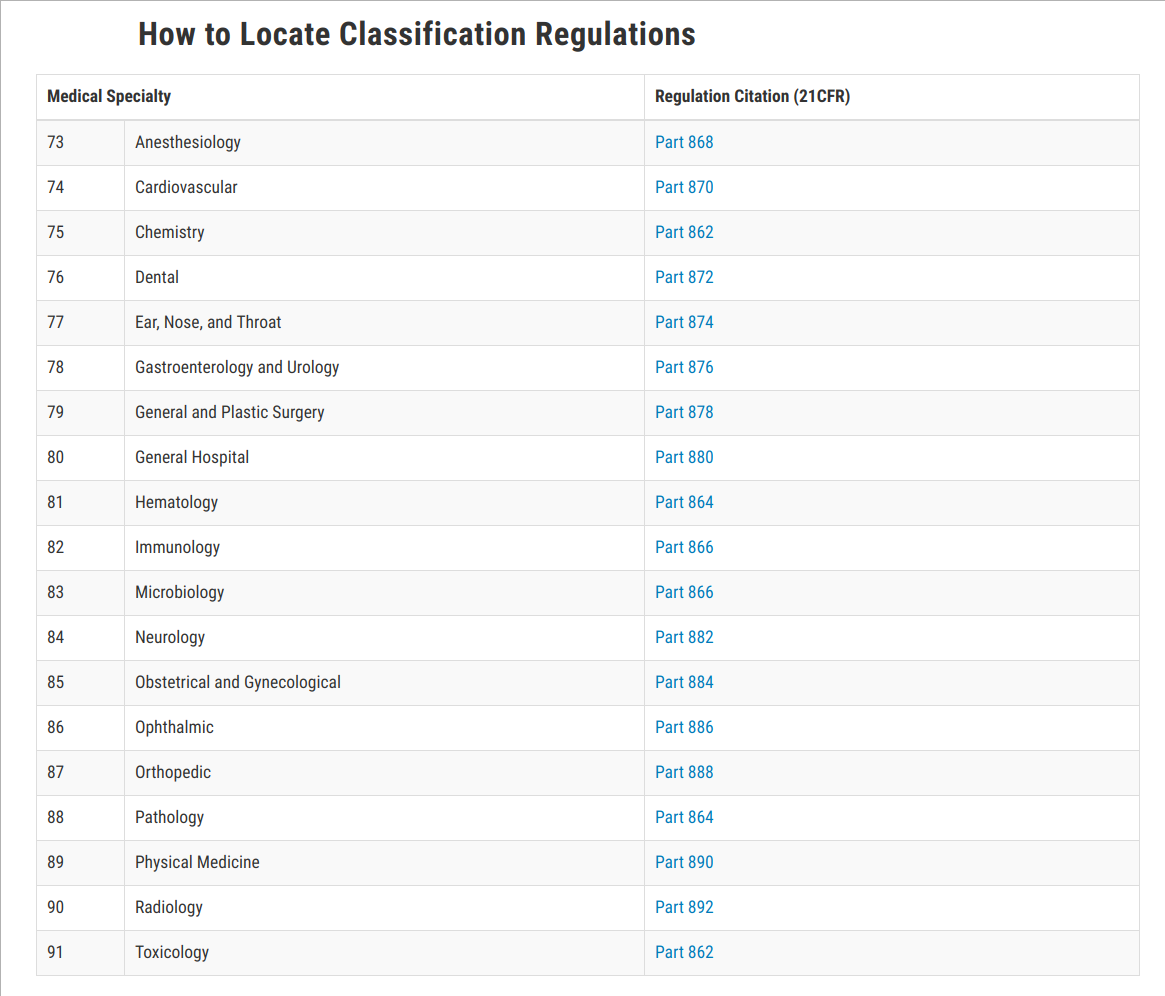
Does An Fda Class 1 Medical Device List Exist

5 Tips For Medical Device Registration Across Global Markets

Product Launch Medical Marketing
Guidance Document Software As A Medical Device Samd Classification Examples Canada Ca

Cbc Takes You Inside Health Canada S Medical Device Records Medical Medical Device Healthcare Provider
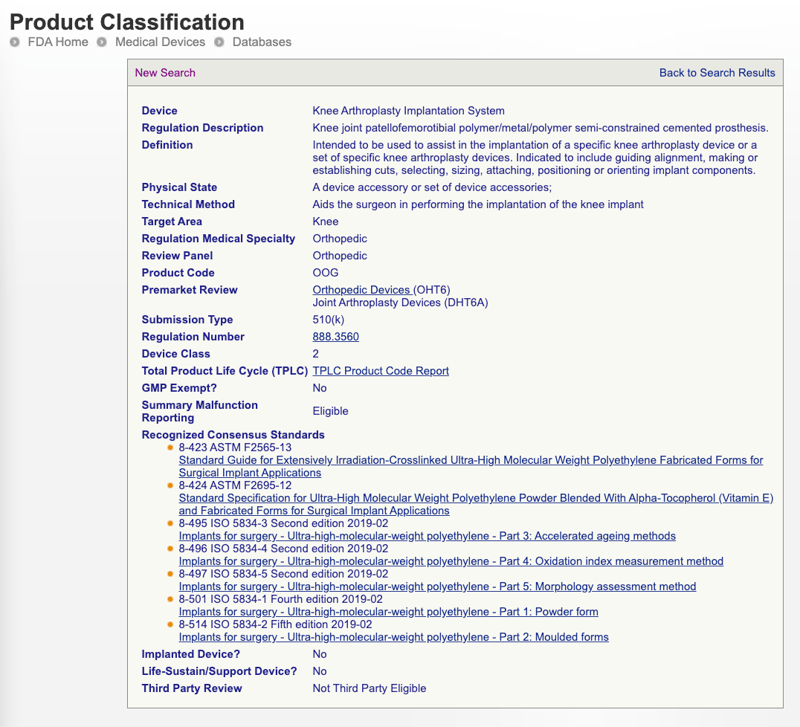
Complete Guide To Bringing A Medical Device To Market

Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca

Medical Contact List Template Dotxes Contact List Template Contact List List Template

Pin On Charts Graphs And Figures

Full List Of Medical Conditions That Qualify For Disability Tax Credit Bmdservices Ca Medical Conditions Medical Conditioner

Free Job Posting Sites In Canada 25 Best Websites For Canadian Jobs Ads2020 Marketing Job Posting Job Posting Sites Free Job Posting

Adventa Recruiting Now Accommodation Free Visa Ticket Benefits Click Here To Apply New Job Vacancies Relationship Management New Job


Posting Komentar untuk "Health Canada Medical Device Listing"