Health Canada Medical Device Licence
103842 an advanced cannabis vaporizer device for medical use. A person outside of Canada sellingmedical devices into Canada is also considered to be a distributor.
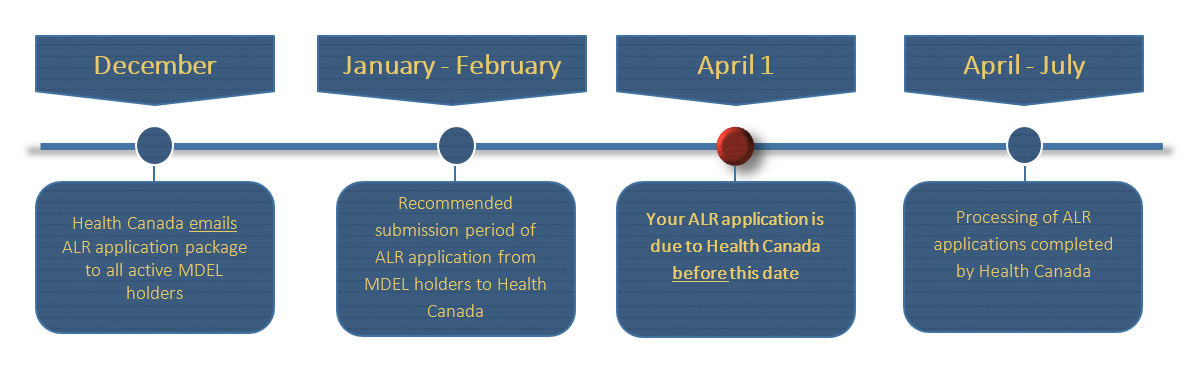
Frequently Asked Questions Medical Device Establishment Licensing And Fees Canada Ca
3 rows Get full assistance to make import distribute medical devices and meet ISO 13485 or 13488.

Health canada medical device licence. A link button or video is. Establishment Licensing EL fees Health Canada inspects establishments to assess whether they comply with regulatory requirements to conduct regulated activities related to drugs and medical devices. Guidance documents are designed to be living documents and will be revised as necessary.
Guidance documents have been prepared to assist in the interpretation of policies and governing statutes and regulations. Medical device licensing. Manufacturers of medical devices must hold a medical device licence to import or distribute a Class II III or IV medical device in Canada.
Please select all that apply. Medical Devices Active Licence Listing MDALL Purchase of Licensed Medical Devices for Use in Health Care. Announce it has been issued a Medical Device Establishment License MDEL from Health Canada.
The MDEL is a license issued to companies for the activities of manufacturing importing and distributing selling of all four classes of medical devices for human use in Canada. An MDEL provides Health Canada assurance that medical devices sold or imported into Canada meet the safety requirements set out in the Medical Devices. A Medical Device Establishment Licence MDEL is a licence issued to Class I manufacturers as well as importers or distributors of all device classes to permit them to import or distribute a medical device in Canada.
Access forms and guidance documents to help you apply for a medical device licence. Primo Nutraceuticals Inc. Health Canada Medical Device License MDL A Canadian Medical Device License MDL is required for companies selling Class II - IV medical devices in Canada.
Obtaining an MDL is comparable to the US FDA 510 k process. An MDEL is issued by the Inspectorate based on an establishment certifying that they meet certain requirements and are then inspected for compliance. A Medical Device Establishment Licence MDEL is separate from a Medical Device Licence and is issued for the activities of importing and selling medical devices for human use in Canada.
Only Class I devices require an establishment license. Device Licenses for Ultrasonic Diagnostic Systems and Transducers Health Canada the countrys regulating authority in the sphere of medical devices and other healthcare products has published a guidance document dedicated to medical device license applications for ultrasound diagnostic systems and transducers. Person other than a manufacturer an importer or a retailer whosells a medical device in Canada for the purpose of resale or useother than for personal use.
Application for a New Medical Device Licence for a Private Label Medical Device 2020-04-01 Bed-related Entrapment and Fall Report Form 2008-03-17 Class II Medical Device Licence Amendment Application Form. A Canadian Medical Device License is a license to distribute medical devices. Therefore launching a new product in Canada is one of the fastest ways for start-up medical device companies to.
Report a problem or mistake on this page. Before a drug or medical device is authorized for sale in Canada Health Canada reviews it to assess its safety efficacy and quality. They are intended to assist in preparing the various device licence applications required when seeking an authorization to sell a medical device product in Canada.
Canadian Medical Device Licensing is generally a more straightforward process than the 510 k submission process for the US FDA and the European CE Marking Process. Also search for a licensed device using the listing database. The MDL is a product approval while a MDEL is a permit for the companydistributorimporter itself.
SMITHS FALLS ON and TUTTLINGEN Germany April 3 2020CNW Storz Bickel is pleased to announce that Health Canada has issued a Medical Device Licence for the new Volcano Medic 2 License No. PDF fillablesaveable 247 K 2020-04-15 Doc Version - 249 K Class IV Medical Device Licence Amendment Application Form. Medical Devices Licence Listings.
In the late 1990s Health Canada was given the authority under the Financial Administration Act to charge industry user fees in order to recover some of the costs related to service delivery for medical devices. Therefore your company will be able to sell directly to physicians prescribing your device if you have a Class II III or IV Medical Device License.
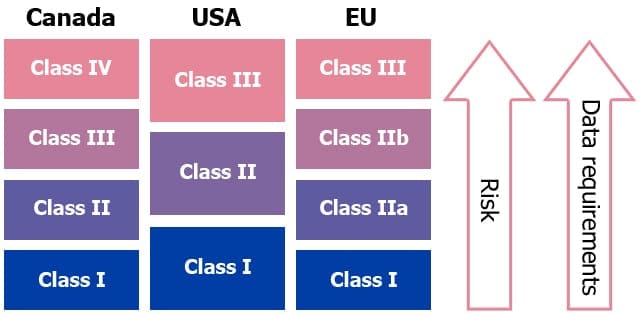
Medical Device Regulations Classification Submissions
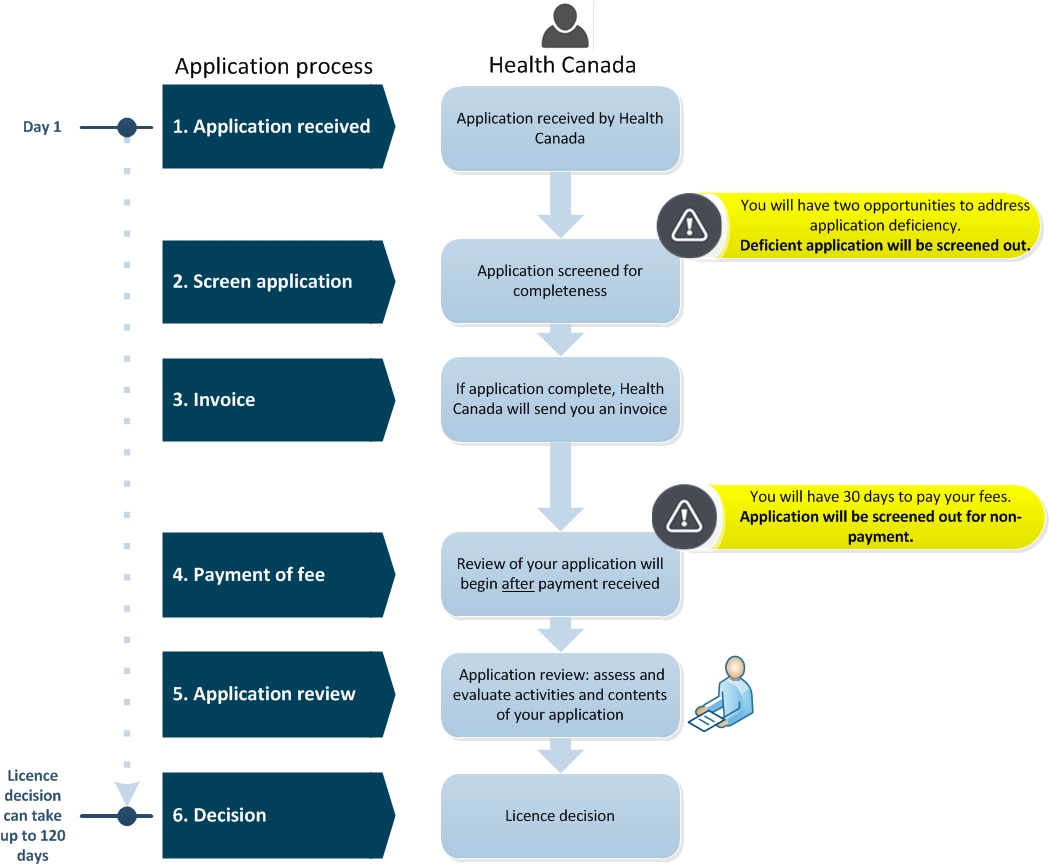
Frequently Asked Questions Medical Device Establishment Licensing And Fees Canada Ca
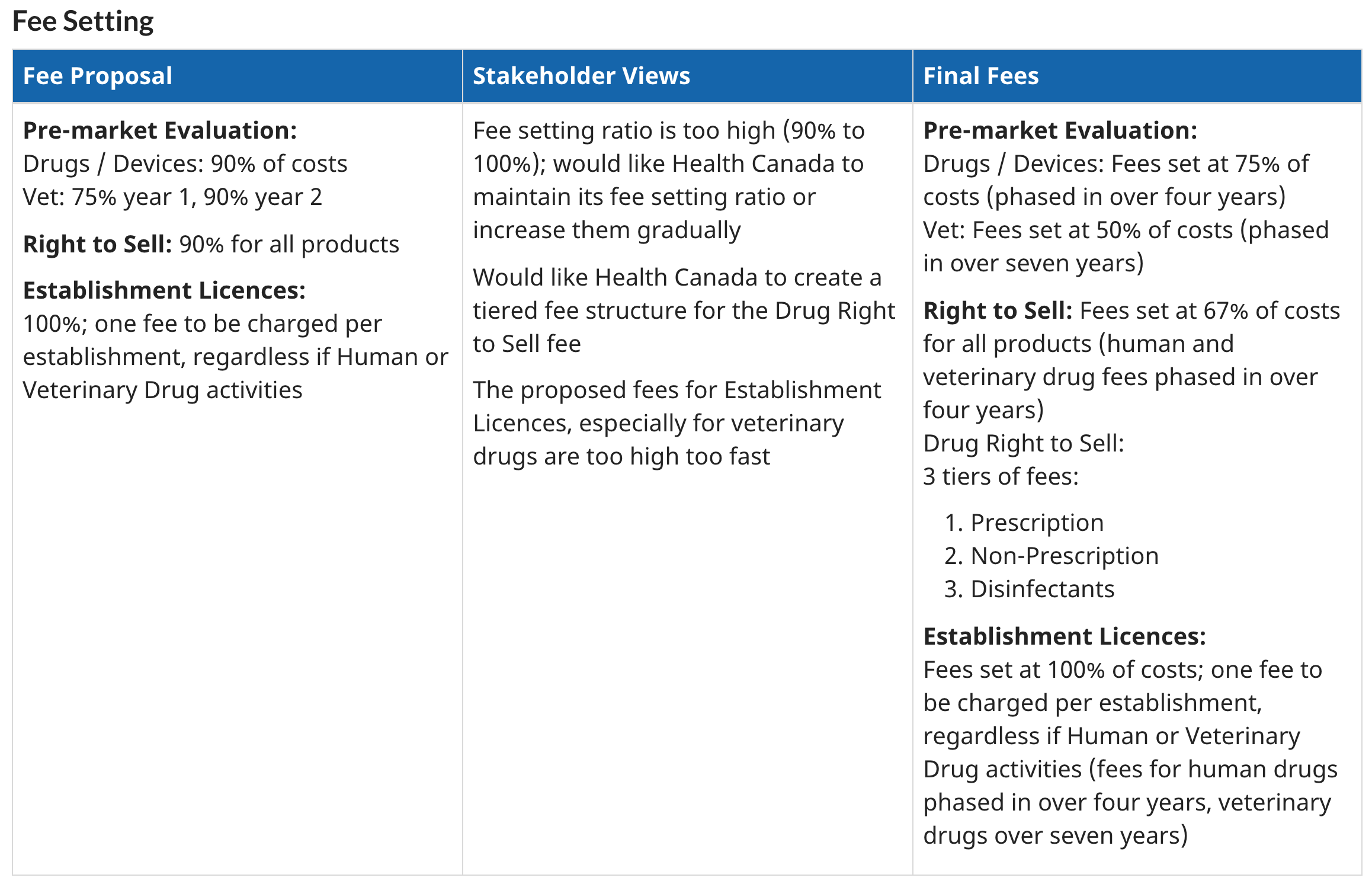
Health Canada Increases Regulatory Prices Of Medical Devices Regdesk

Canada Medical Device Approval Chart Emergo
Https Globi Reg Com Wp Content Uploads 2019 10 Frequently Asked Questions Medical Device Establishment Licensing And Fees Pdf
Huron Receives Health Canada Medical Device License For Tissuescope Le Huron Digital Pathology

Covid 19 Related Medical Device Approvals In Us Canada And Eu
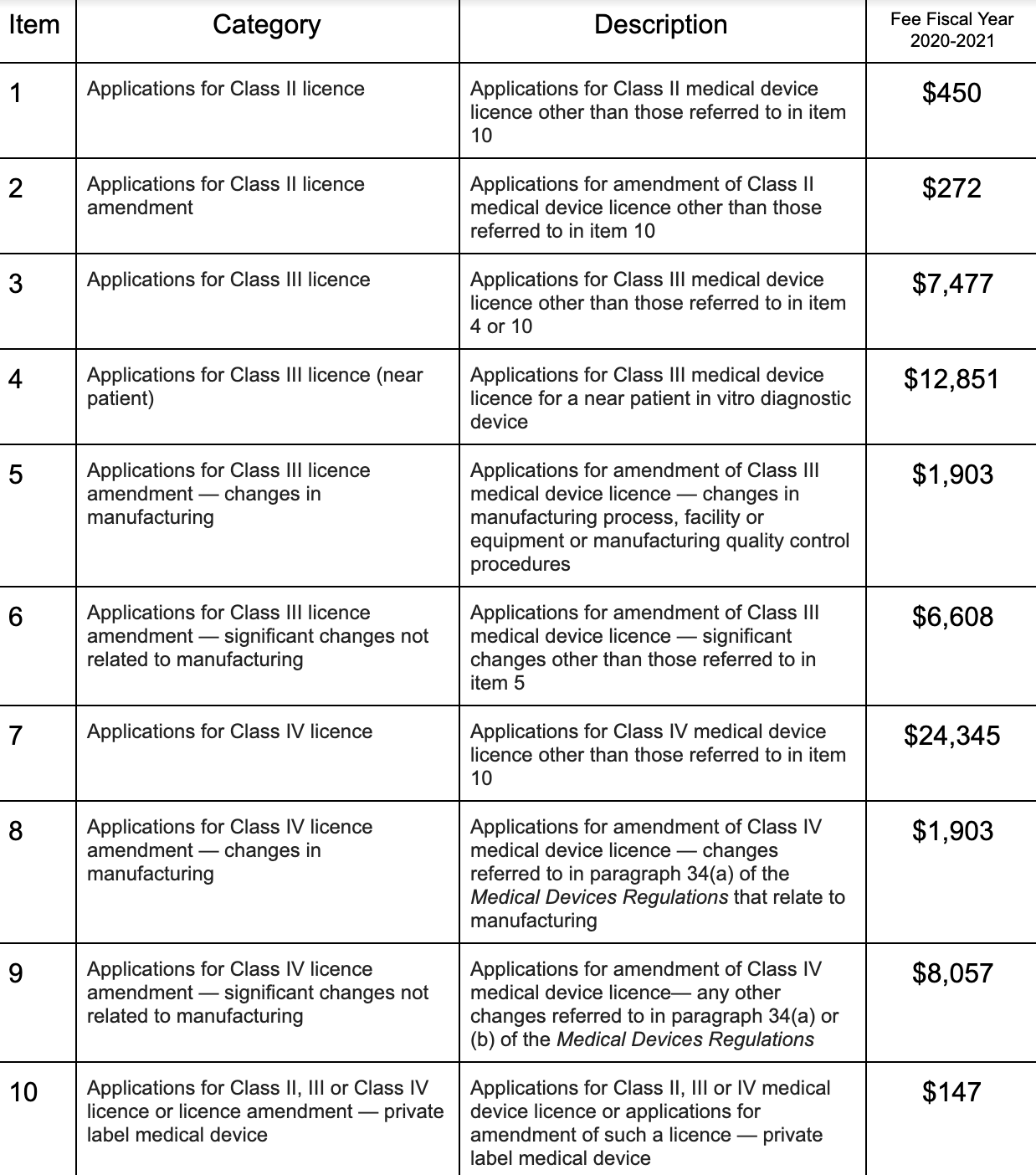
Health Canada Increases Regulatory Prices Of Medical Devices Regdesk

4 Health Canada S Medical Device Classification Under The Food And Download Table

Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca
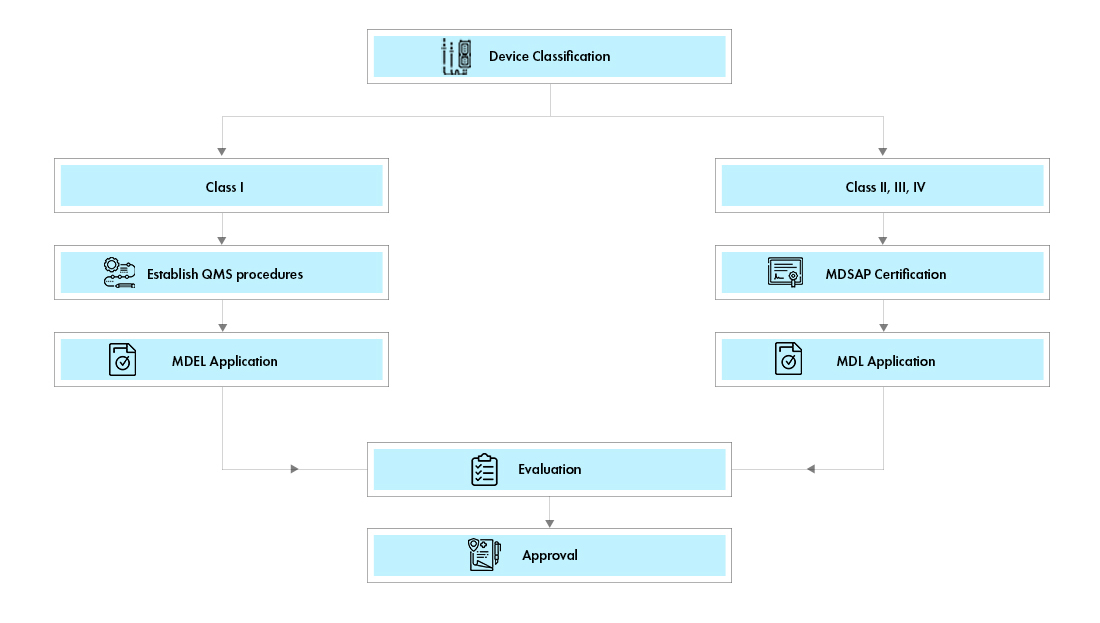
Medical Device Registration Canada Health Canada Mdsap Certification Mdel
Class Ii Iv Medical Device Investigational Testing In Canada Vantage Biotrials
Class Ii Iv Medical Device Investigational Testing In Canada Vantage Biotrials
Https Www Fdanews Com Ext Resources Files 2018 2 11 29 18 Healthcanada Pdf 1543529549

Health Canada Medical Device Licence For Mexico Information

Health Canada Increases Regulatory Prices Of Medical Devices Regdesk

Beyes Dental Handpiece And Equipment

Beyes Dental Handpiece And Equipment

Posting Komentar untuk "Health Canada Medical Device Licence"