Health Canada Api
Canadian Nutrient File API Database The Canadian Nutrient File CNF is the standard reference food composition database reporting the amount of nutrients in foods commonly consumed in Canada. DOSSIERs Finished Dosage.
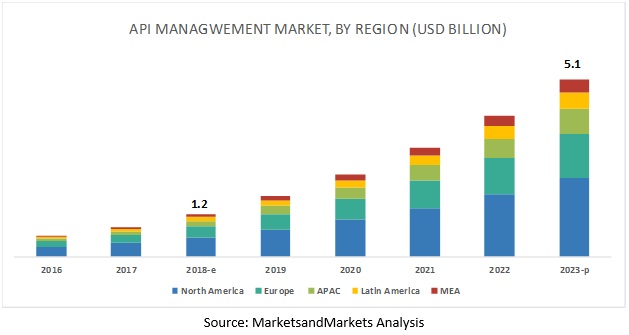
Api Management Market By Solution Services 2023 Marketsandmarkets
The database is managed by Health Canada and provides a source of information about Canadian clinical trials involving human pharmaceutical and biological drugs.

Health canada api. The following APIs serve JSON responses and will require an API key to access outside the canadaca domain. Any requests are made relative to this URI. It ensures that high-quality health services are accessible and works to reduce health risks.
29 Suppliers 21 USDMF 4 CEPCOS 5 JDMF 2 EU WC 7 Listed Suppliers. Coronavirus disease COVID-19 Outbreak update. Health Canada is notifying stakeholders of the interim approach concerning the implementation of Drug Establishment Licensing requirements for certain Active Pharmaceutical Ingredients APIs used in the fabrication of human and veterinary pharmaceutical drugs that are also used outside of the pharmaceutical industry.
Through a phased in approach Health Canada will populate the remaining NOCs for Veterinary drugs from January 1 1994 to September 18 2000. Documentation for the Medical Devices Active Licence Listing MDALL API. An Active Ingredient is any component that has medicinal properties and supplies pharmacological activity or other direct effect in the diagnosis cure mitigation treatment or prevention of disease or to.
We are a federal institution that is part of the Health portfolio. This Application Programming Interface API allows. Approved Drug Products containing Potassium Chloride API listed with Health Canada.
The selection of a particular compound as the API starting material and its. APIs Active Pharmaceutical Ingredients. API quality directly impacts the safety and efficacy of the finished pharmaceutical dosage form.
Canadian input has been. Health Canada considers fabrication packaginglabeling and testing of sterile APIs not terminally sterilized as being finished dosage form manufacture and therefore these guidelines only apply to the manufacture of sterile APIs up to the point immediately prior to the APIs being rendered sterile. This was because the nitrosamine impurity N-nitrosodimethylamine NDMA was found in the active pharmaceutical ingredient API.
An example of an API is the acetaminophen contained in. Health Canadas Clinical Trials Database is a listing of information about phase I II and III clinical trials in patients. An active pharmaceutical ingredient API is an active ingredient or raw material used in the fabrication of a pharmaceutical drug dosage form.
An API starting material is proposed by the applicant and assessed by Health Canada to determine whether the controls on the drug substance eg. Health Canada is responsible for helping Canadians maintain and improve their health. The extension of Good Manufacturing Practices GMP to Active Pharmaceutical Ingredients APIs has increasingly been recognized as a necessary element in ensuring the overall quality and consistency of marketed drug products.
In the summer of 2018 several medications containing the active ingredient Valsartan were recalled in Canada and elsewhere in the world. Active ingredients are the substances in drugs that are responsible for the beneficial health effects experienced by consumers. DOCUMENTATION INTRO --- The Health Canada APIs provide an application independent method of querying several raw Health Canada datasets and data sources.
The active ingredient in a pharmaceutical drug is called an active pharmaceutical ingredient API. The base URI for the Drug Product Database is httpshealth-productscanadacaapidrug and you can add parameters to it. Impurities and drug substance manufacturing process eg.
Approved Drug Products containing Polyvinyl Alcohol API listed with Health Canada. For this reason the International Conference on Harmonisation ICH formed a working group in 1997 to develop a GMP guidance for APIs. Control strategy critical process controls intermediate testing can provide appropriate control of quality.
Open Banking Banking Apis Explained W Examples

Api Sourcing The Supply Side For Us Marketed Drugs

List Of Countries Using Google And Apple S Covid 19 Contact Tracing Api
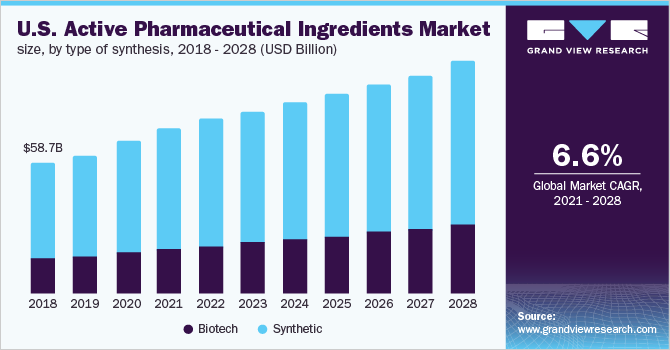
Active Pharmaceutical Ingredients Market Report 2021 2028

Active Pharmaceutical Ingredients Market Size Share Industry Overview 2021 To 2026 With Covid Impact Mordor Intelligence
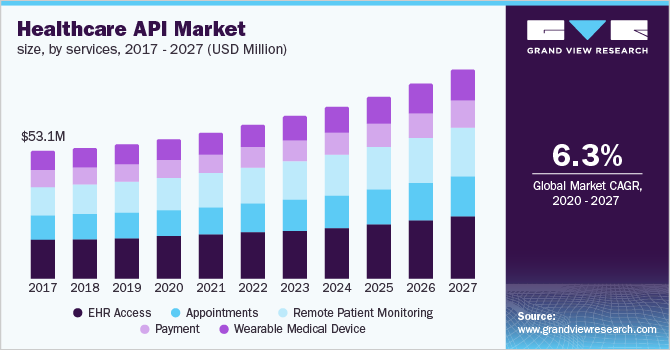
Healthcare Api Market Size Industry Report 2020 2027
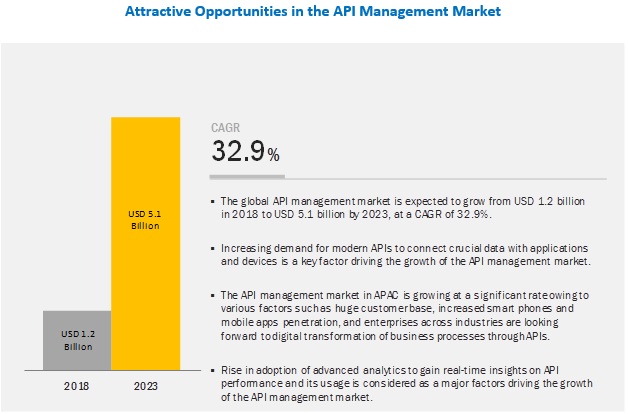
Api Management Market By Solution Services 2023 Marketsandmarkets
Open Banking Banking Apis Explained W Examples

Postman Covid 19 Api Resource Center
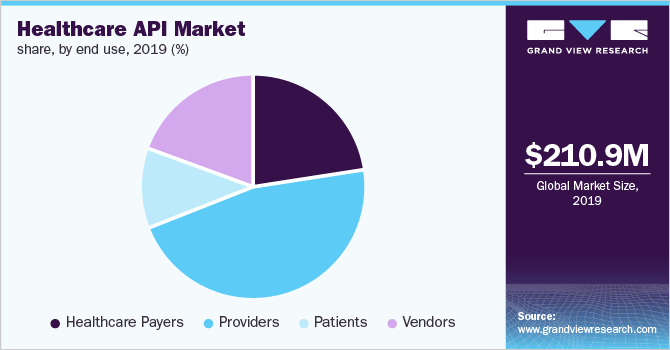
Healthcare Api Market Size Industry Report 2020 2027
Open Banking Banking Apis Explained W Examples

Ibm Api Connect Test And Monitor Test And Monitor United Kingdom Ibm

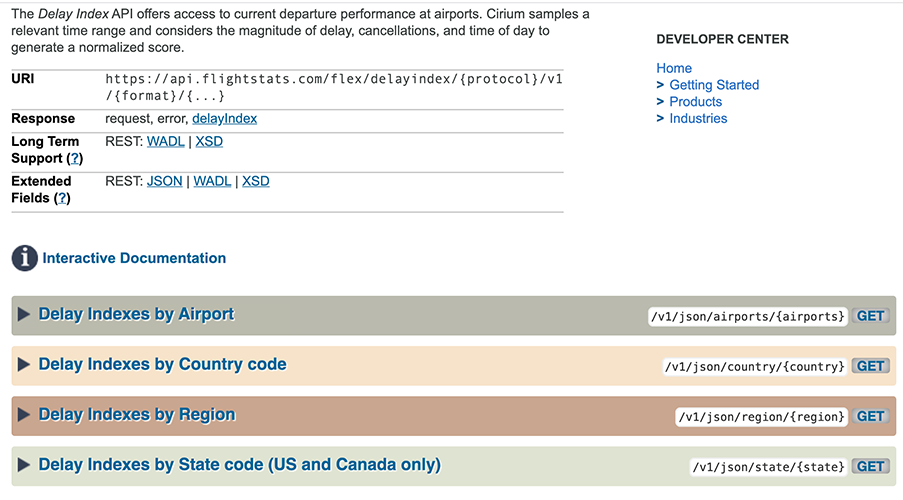


Posting Komentar untuk "Health Canada Api"