Health Canada Nds
An Abbreviated New Drug Submission must be approved by Health Canada the countrys federal department in charge of national health care under. The headline change is in the name.

Health Canada Guidance For Biotechnology Products Professor Peiva
SANDS Supplement to Abbreviated New Drug Submission and SNDS Supplement to a New Drug Submission are types of submission towards Health Canada which stand as important resources to ANDS Abbreviated New Drug Submission and NDS New Drug Submission.

Health canada nds. The FDR amendments are anticipated to remain in effect indefinitely pending the status of the pandemic. Most health products including drugs to be marketed or sold in Canada are reviewed and authorized by the Health Products and Food Branch HPFB of Health Canada more precisely under the Therapeutic Product Directorate TPD or the Biologic and Genetic Therapies Directorate BGTD for drugs and biologic respectively. They are intended to assist in preparing drug submissions when seeking an approval to sell a pharmaceutical drug product in Canada.
Health Canada has retired the subsequent-entry biologics moniker. Canadian Blood Services is a not-for-profit charitable organization. Health Canada has revised its guidance document on the approval pathway for biosimilar biologic drugs biosimilars.
Global Market Access Solutions New Drug Submissions NDS and New Drug Applications NDA Companies looking to launch a new pharmaceutical drug in Canada must first file a New Drug Submission NDS with Health Canadas Therapeutic Products Directorate TPD. Guidance documents have been prepared to assist in the interpretation of policies and governing statutes and regulations. For industry information about COVID-19 visit our COVID-19 Drugs and vaccines section.
Health Canada NDS New Drug Submission is the process through which new drugs are approved and controlled by the Canadian Health Authority before entering the Canadian market. Saudi Food and Drug Administration. Canadian Blood Services was founded in 1998 based on recommendations from the Krever Report on the tainted blood scandal of the early 1990s.
New Drug Submission NDS Supplement to a New Drug Submission SNDS Supplement to a New Drug Submission Confirmatory SNDS-C Abbreviated New Drug Submission ANDS Supplement to Abbreviated New Drug Submission SANDS eCTD. To understand SANDS and SNDS better let us understand ANDS and NDS first. Health Canada will conduct a preliminary assessment while the drug is under review NDS and the sponsor will be notified of the outcome.
Health Canada is responsible for helping Canadians maintain and improve their health. We are a federal institution that is part of the Health portfolio. In Canada new drugs are regulated under Part C Division 8 of Food and Drugs Regulations.
NDS New Drug Submission is the process through which new drugs are approved and controlled by the Canadian Health Authority before entering the Canadian market. In the US a New Drug Application NDA must be submitted to the FDA. These products continue to be approved for sale in Canada during this transition period and are clearly identified in the applicable row.
Consequently a subsequent-entry manufacturer is not allowed to file a submission for a generic drug for the first six years of the eight-year period. Initial submissions Responses Variations. It ensures that high-quality health services are accessible and works to reduce health risks.
In some cases applicants have filed a new drug submission under the Food and Drug Regulations to transition a product previously authorized under the Interim Order Respecting the Importation Sale and Advertising of Drugs for Use in Relation to COVID-19. NDS vs ANDS In Canada there is no equivalent to the 505 b 2 pathway a new drug must be filed as either a New Drug Submission NDS or an Abbreviated New Drug Submission ANDS or generic submission. Coronavirus disease COVID-19 Outbreak update.
CBS is regulated as a biologics manufacturer by Health Canada and primarily funded by the provincial and territorial ministries of health. Health Canada anticipates that the FDR amendments will be in effect by March 16 2020 in order to give manufacturers 6 months to file and obtain their NOC prior to the expiry of the Interim Order for Drugs and Vaccines on September 16 2021. Health Canada reserves the right to request information or material or define conditions not specifically described in this document in order to allow the Department to adequately assess the safety efficacy or quality of a therapeutic product.
In Canada new drugs are regulated under Part C Division 8 of Food and Drugs Regulations.

Health Canada Forms Pdf Templates Download Fill And Print For Free Templateroller

Health Canada Guidance For Biotechnology Products Professor Peiva
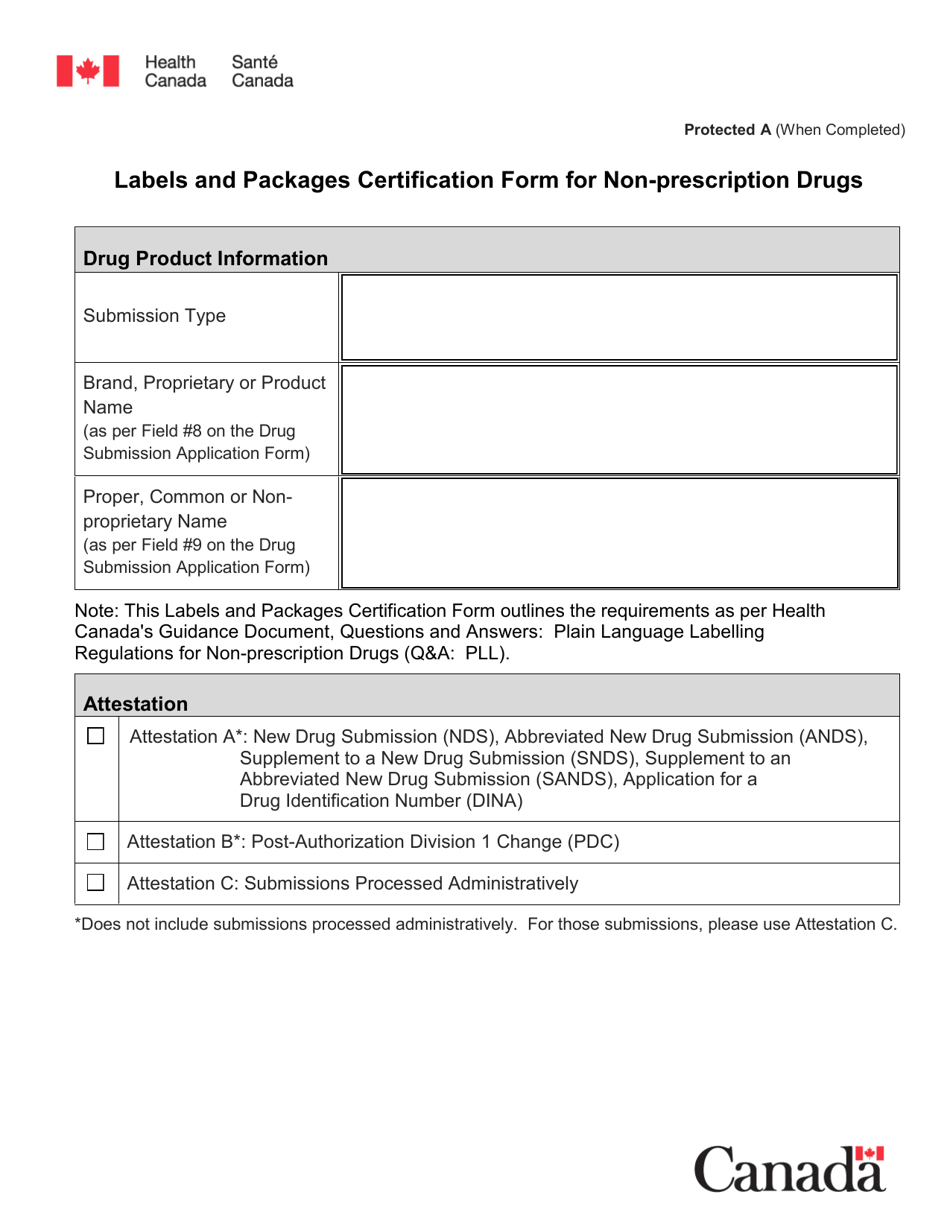
Canada Labels And Packages Certification Form For Non Prescription Drugs Download Fillable Pdf Templateroller
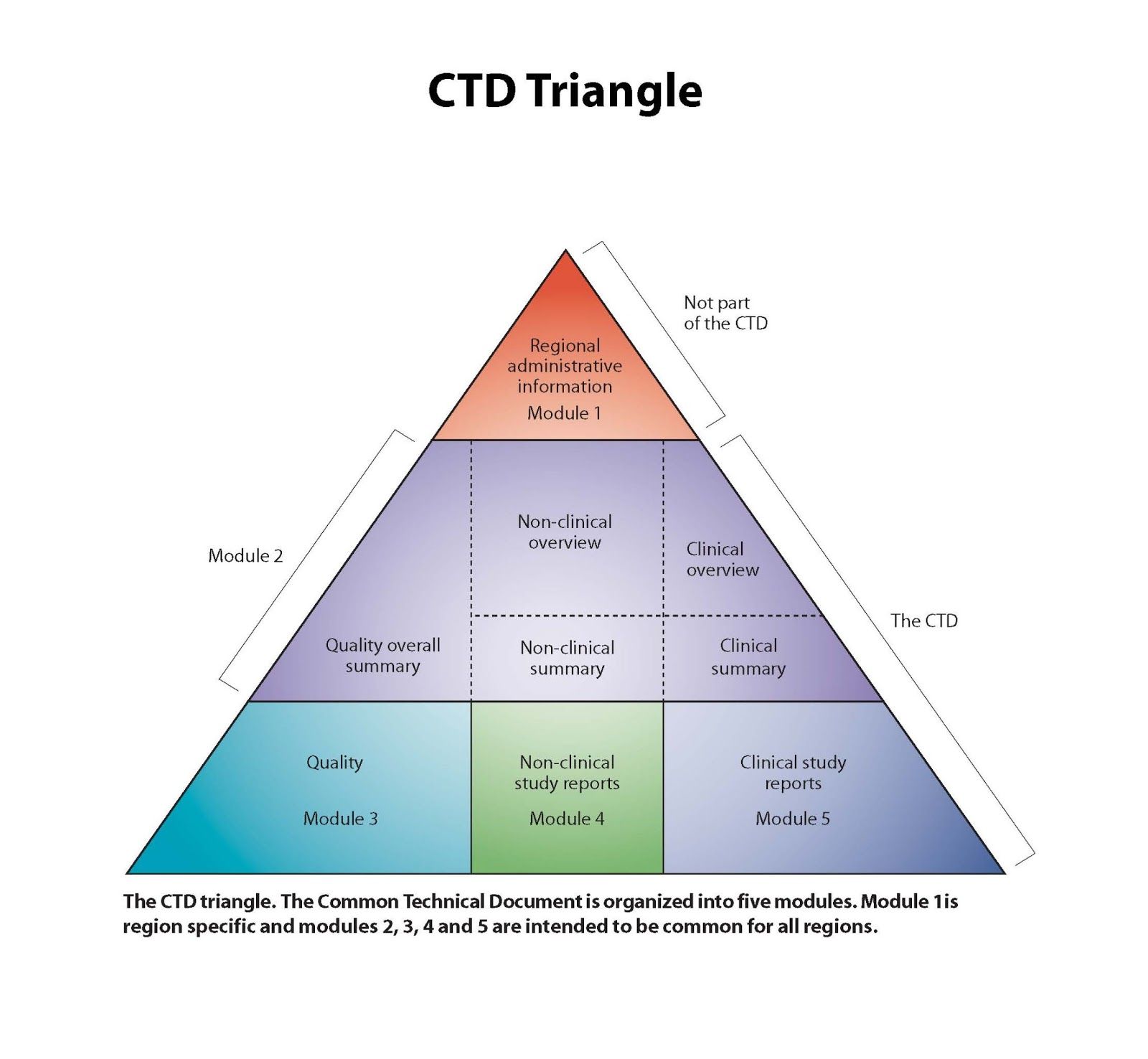
The Drug Review And Approval Process In Canada An Eguide Spharm Canada S Drug Regulatory Experts

Health Canada Expands The Scope Of Regulatory Activity Types For Mandatory Use Of Ectd

The Drug Review And Approval Process In Canada An Eguide Spharm Canada S Drug Regulatory Experts
Health Canada Guidance For Biotechnology Products Professor Peiva

Summary Of Health Canada S Public Release Of Clinical Information Initiative

How To Navigate Health Canada Xml Pm Requirements Reed Tech
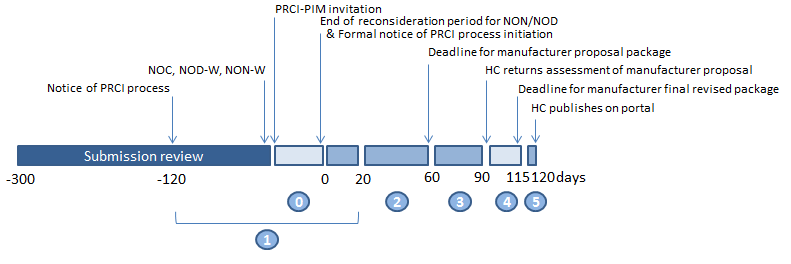
Public Release Of Clinical Information Guidance Document Canada Ca
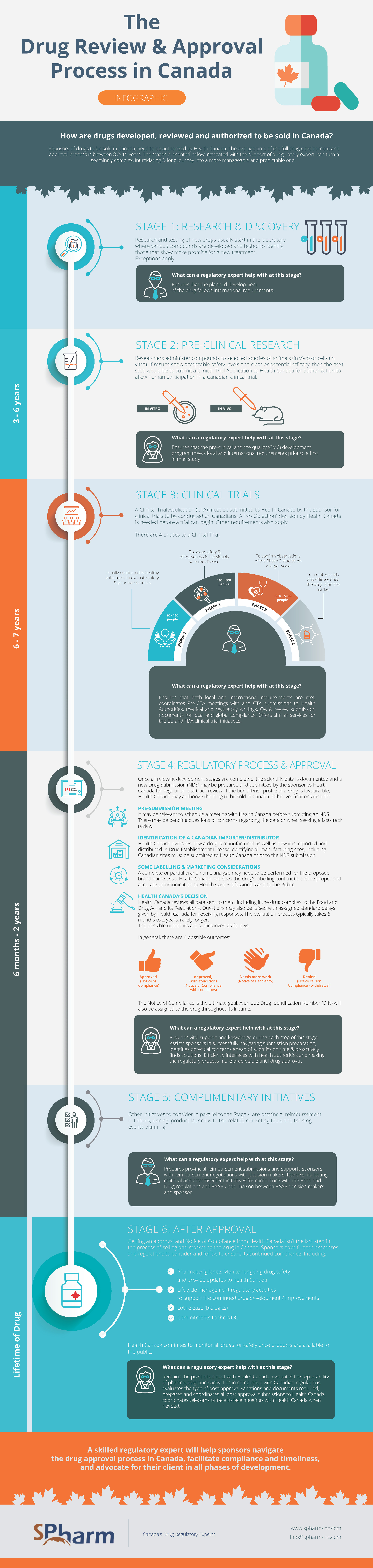
The Drug Review And Approval Process In Canada An Eguide Spharm Canada S Drug Regulatory Experts

Health Canada Guidance For Biotechnology Products Professor Peiva
Http Www Biotech Ca Wp Content Uploads 2016 04 Vaccines 4 2010 1 Pdf
Https Www Canadianinstitute Com Pharma Patents 373l17 Tor Wp Content Uploads Sites 1707 2016 11 Difranco 1000am Day1 Pdf
Https Ntp Niehs Nih Gov Iccvam Methods Biologics Vaccine Canada Lotreleaseprgm Pdf

Health Canada Form Hc Sc 3011 Drug Submission Application
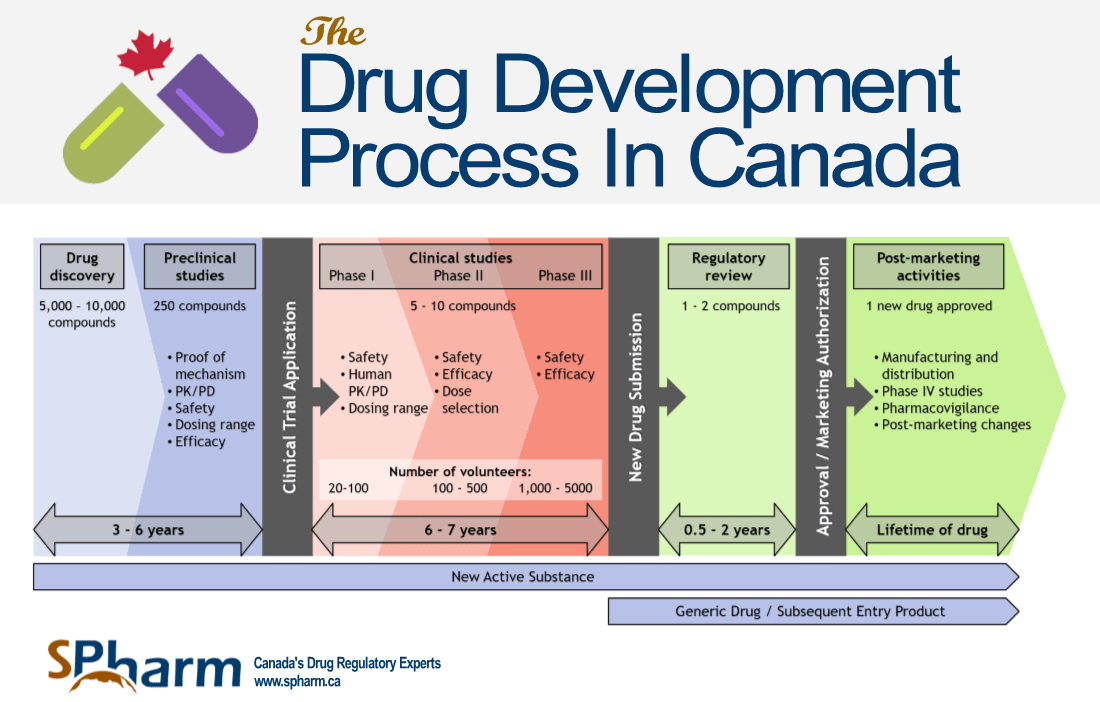
The Drug Review And Approval Process In Canada An Eguide Spharm Canada S Drug Regulatory Experts

The Drug Review And Approval Process In Canada An Eguide Spharm Canada S Drug Regulatory Experts

Mock Up Labels And Packages Certification Form For Prescription Products
Posting Komentar untuk "Health Canada Nds"