Medical Device Establishment Licence Health Canada
Medical gown manufacturers authorized in Canada are listed in the Medical Devices Establishment Licence Listing database. On receipt of the application applicants would be in receipt of the system generated auto-response.

2012 Form Canada Frm 0292 Fill Online Printable Fillable Blank Pdffiller
Establishment Licensing EL fees Health Canada inspects establishments to assess whether they comply with regulatory requirements to conduct regulated activities related to drugs and medical devices.
Medical device establishment licence health canada. Before a drug or medical device is authorized for sale in Canada Health Canada reviews it to assess its safety efficacy and quality. All Canadian drug establishments must hold since January 1 1998 an establishment licence to fabricate package label distribute import wholesale or test a drug. Health Canada will be expediting all regulatory submissions related to COVID-19 medical devices.
Drug establishment licence fees Complete the application form for a drug establishment licence and access related guidance documents including information on required fees. Also search for a licensed device using the listing database. Diagram 1 is a breakdown of the steps in the 250 days of the drug establishment licence application timeline.
Guidance documents to help with licensing a site or establishment for drugs blood medical devices or cells tissues and organs. HOW LONG WILL IT TAKE TO GET A PRODUCT LICENSE. Management of Applications and Performance for Drug Establishment Licences GUI-0127 Drug Establishment Licence Application Forms and Instructions FRM-0033.
Information on how to obtain a Medical Device Establishment Licence for medical gowns. Fast-tracking approval of medical gowns in Canada. MDEL Bulletin January 22 2021 from the Medical Devices Compliance Program.
This requirement came into effect January 1 1999. You will be invoiced the full fee if you do not apply for and receive small business status before submitting your submission application notification. Medical device licensing.
Ancillare the industry leader in Clinical Trial Ancillary Supply Chain CTASC obtains authorization to distribute medical devices in Canada. Also access listings for establishment licences. Yes section 44 of the Medical Devices regulations states that no person shall import or sell a medical device unless they hold an establishment licence.
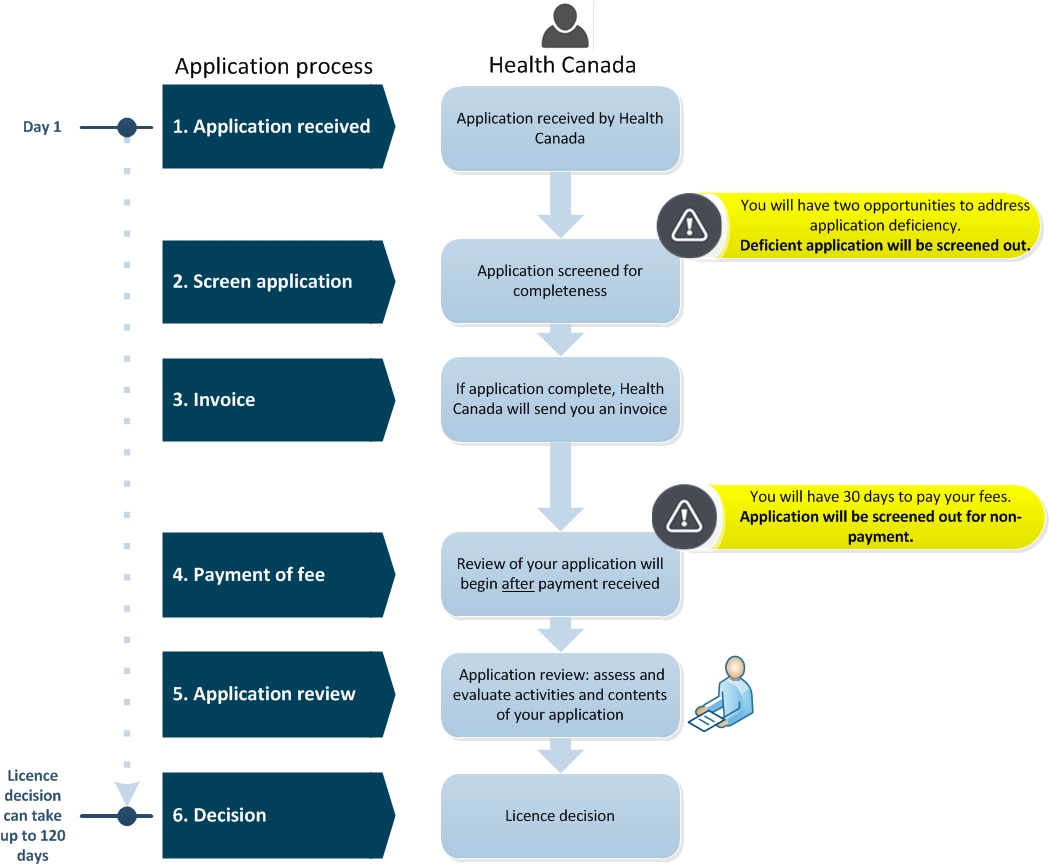
For example Health Canada is targeting 20 days for Class III devices and 25 days for Class IV devices after receipt of required information. To receive a fee reduction you must first apply for and receive small business status for each unique identifier. Canada is also speeding up the importation and sale of medical devices used to.
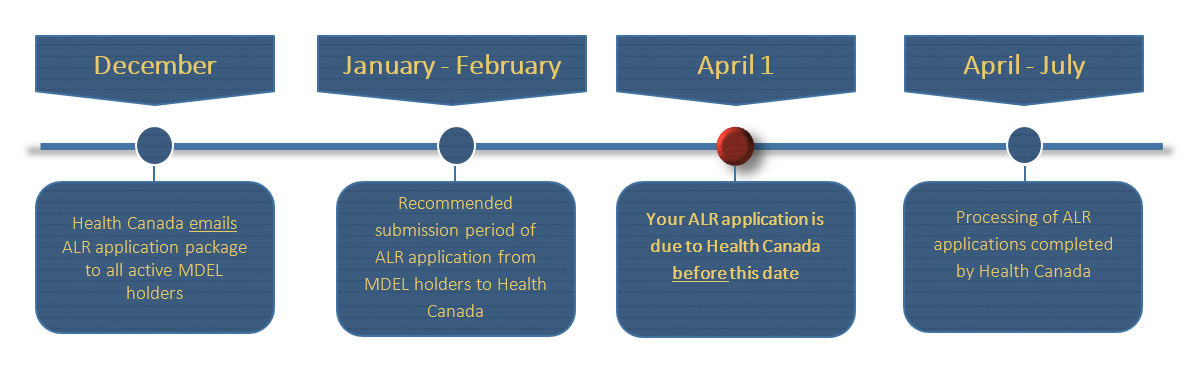
In this webinar youll learn how to complete sign and submit your medical device establishment licence MDEL ALR application. License an establishment or site for drugs or health products. An MDEL provides Health Canada assurance that medical devices sold or imported into Canada meet the safety requirements set out.
As of April 1 2020 Health Canada will adopt measures for small business fee reductions for human and veterinary drugs and medical devices. Access forms and guidance documents to help you apply for a medical device licence. Receipt of an application marks day zero of Health Canada days.
The Medical Device Establishment Licensing Unit MDELU invites you to a free webinar session on the annual licence review ALR. Ancillare obtained its Medical Device Establishment License MDEL strengthening its global distribution network through the ability to import registered medical devices for clinical use in Canada. Medical device establishment fees Instructions for filling out the medical device establishment licence application.
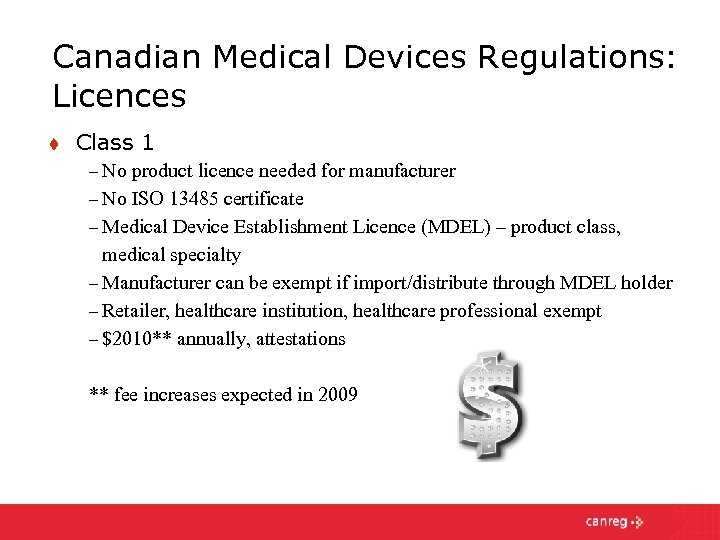
A Medical Device Establishment Licence MDEL is a licence issued to Class I manufacturers as well as importers or distributors of all device classes to permit them to import or distribute a medical device in Canada.
Frequently Asked Questions Medical Device Establishment Licensing And Fees Canada Ca

Entering The North American Market The Regulatory Landscape
Https Globi Reg Com Wp Content Uploads 2019 10 Frequently Asked Questions Medical Device Establishment Licensing And Fees Pdf

Certifications Registrations Mountain Integrated Medical Devices

Beyes Dental Handpiece And Equipment

Toefx Granted Medical Device Establishment License From Health Canada The Forge

Dsa Consultants Drug Establishment Licenses

Canada Medical Device Approval Chart Emergo
Entering The North American Market The Regulatory Landscape

Canada Medical Device Approval Chart Emergo

Health Canada Mdel Application Cansummit Canadian Medical Devices Market Consultants

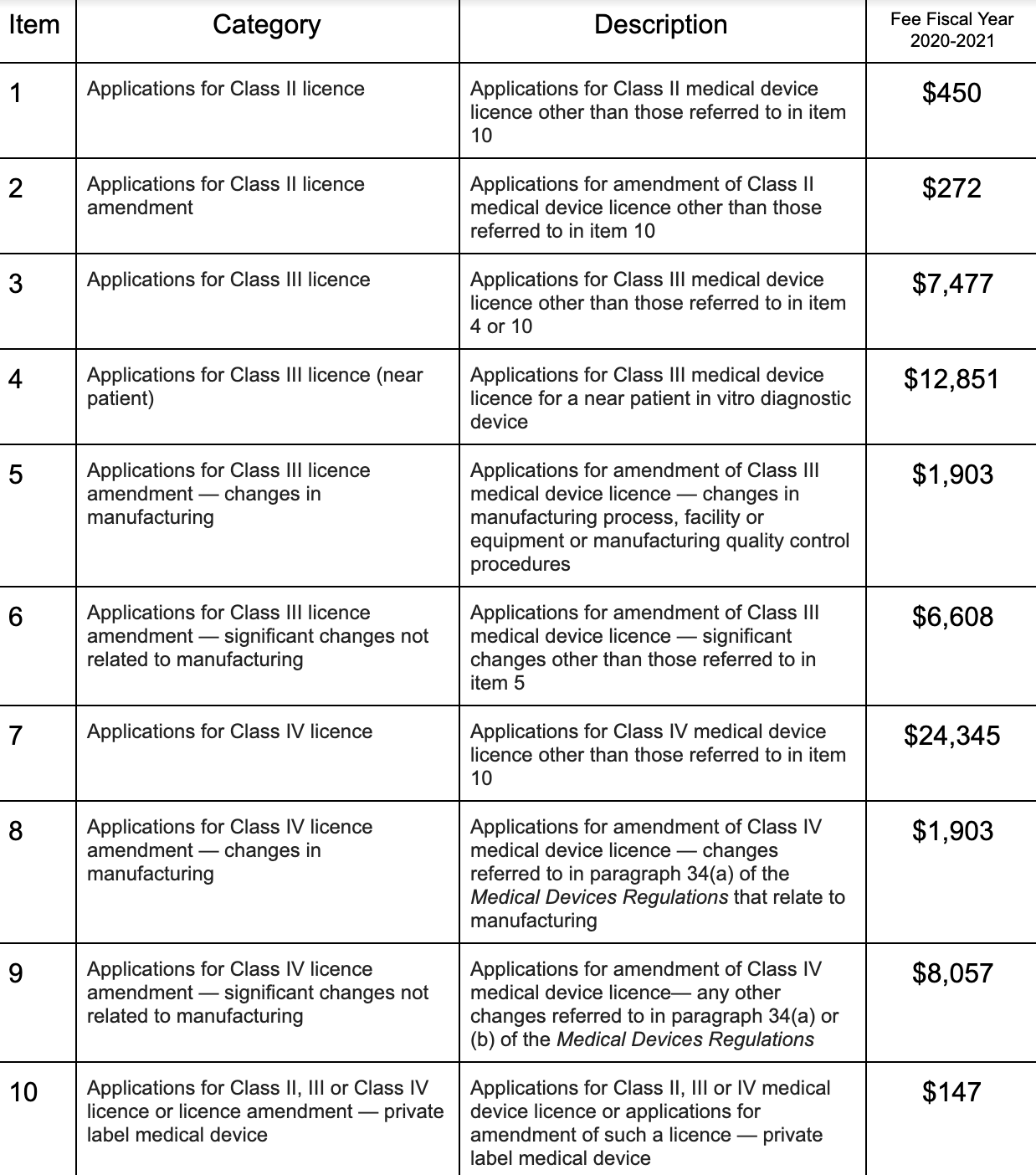
Health Canada Updates Fees And Introduces The Small Business

Frequently Asked Questions Medical Device Establishment Licensing And Fees Canada Ca
Health Canada Increases Regulatory Prices Of Medical Devices Regdesk

Covid 19 Related Medical Device Approvals In Us Canada And Eu
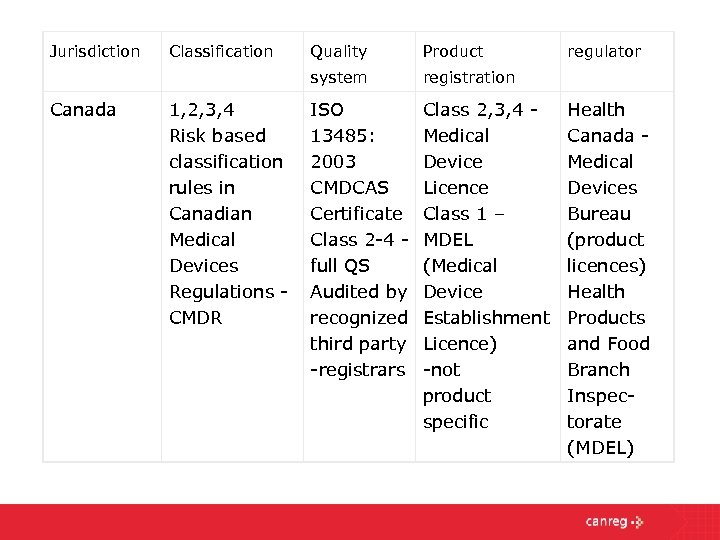
Entering The North American Market The Regulatory Landscape
Guidance On Medical Device Establishment Licensing Mdel Medical Device License


Posting Komentar untuk "Medical Device Establishment Licence Health Canada"