Health Canada Category Iii Medical Assessment
US FDA or CE etc. Corrected vision 69 in the better eye and 615 in the.
Frequently Asked Questions Medical Device Establishment Licensing And Fees Canada Ca
Medical device licence Class III or US FDA.
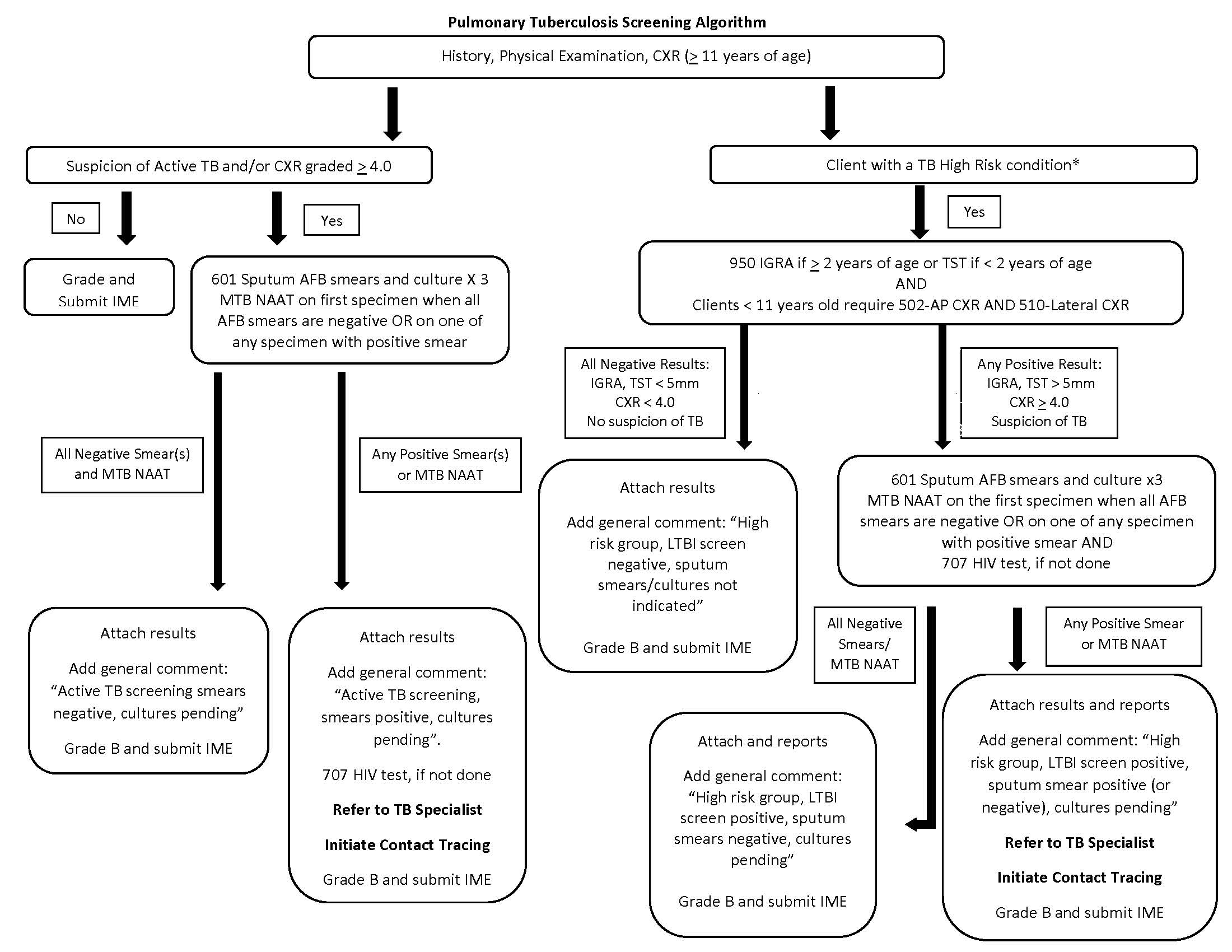
Health canada category iii medical assessment. Medical device classifications of medical devices in jurisdictions outside Canada eg. Has anyone done the Health Canada Category III Medical Assessment for Student Border Officer Jobs or other jobs. Health Canada classifies all medical devices into one of the following four classes ie.
A medical assessment is the review of an applicants medical file by a Citizenship and Immigration Canada CIC medical officer or delegated staff. The classification procedures in Canada and the EU are quite similarmanufacturers must classify their medical devices according to the rules and criteria set out in the relevant medical device regulations Canada and directives EU as indicated in Table 1. This Category III assessment evaluates your physical ability to perform the duties of a border services officer.
Class I lowest risk Class II Class III and Class IV highest risk. What was it like. Medical devices are classified according to Health Canadas risk-based system.
To help manufacturers with this transition Health Canada has introduced changes to the Medical Device. Your most comprehensive health screening. Top medical experts and most advanced technology.
Ad Insight in your health within a day. IVDs are also classified as Class I through IV using a set of 9 rules which can be. Explore our innovative treatments visit us and enjoy a better healthier life.
Security Standards to obtain and maintain a valid Enhanced Security clearance Medical Fitness of the Health Canada Occupational Health Assessment Category III To apply to the Environmental Response student program complete the application online and submit it through the GC Jobs site. From looking online and at the Health Canada website I found the following forms and guidances Class 3 non-in vitro diagnostic devices nIVD new and amendment applications Which breaks down the application content for Class III devices. The product is classified as Class III license type - medical device system.
Class IV Canada generally corresponds to Class III ECD Class III Canada generally corresponds to Class IIb ECD Class II Canada generally corresponds to Class IIa ECD. All manufacturers of class II III and IV medical devices sold in Canada are required to transition to the Medical Device Single Audit Program. You must meet the following vision and hearing standards.
To maintain your medical device licences active you must submit documented evidence of your transition to the Medical Devices Bureau by December 31 2018. Health Canada will assess the reasons for the closure and initiate post-market activities if necessary. De Novo or 510k or PMA.
Part I Posted by Rob Packard on January 2 2015 11 Steps to Obtaining CMDCAS Certification-Part I focuses on the process of verifying the classification selecting a registrar and more. I II III and IV. 2-5 In the US the classifications and ancillary information relating to medical device.
So far Health Canada has accepted ISO 13485 certificates issued by certification bodies accredited by the Standards Council of Canada SCC the national accreditation organisation of Canada and recognised by Health Canada under the Canadian Medical Devices Conformity Assessment. 53 rows All medical information forms and records transmitted or used in connection with these. Class II III and IV medical devices must be licenced prior to importation or sale in Canada.
If the reasons for closure of the MF are related to safety the Applicant should be informed of the reasons and should contact Health Canada regarding the Health Risk Assessment. Make an appointment now. There are four device risk classifications in Canada.
The level of audit may depend on the category andor Class of a medical device and the. Do not apply to Canada. 11 Steps to Obtaining CMDCAS Certification.
A guidance document for device classification is published by Health Canada. Procedures to classify medical devices. There are four device classifications--Class I II III and IV--using a set of 16 rules found in Schedule 1 Part 1 of the Canadian Medical Devices Regulations CMDR SOR98-282.
Category III physical test. Ad We at Poseidonia provide advanced specialized and modern therapies for everyone. Canadian classes of medical devices normally correspond to the European Council Directive 9342EEC MDD devices there may be exceptions.
Medical devices are classified into one of four classes by means of classification rules where Class I represents the lowest risk and Class IV represents the highest risk. Health Canada will not conduct fitness-to-work assessments if an individual is receiving workerscompensation as the workers compensation boards can provide this service. Regulatory Provisions All devices offered for sale in Canada must comply with the Food and Drugs Act.
Regulators assessment bodies for medical devices Irina Tsyganova Director Devices Applications and Verification. A physician designated by Health Canada completes an Occupational Health Assessment Report. Cannot advertise or represent by label a treatment for a Schedule A disease or disorder Section 3 Cannot sell or advertise a device that may cause harm Cannot sell or advertise a device in a misleading or deceptive way All medical devices those used on human beings must also comply.
Validity period of a medical certificate The validity period is 12 months from the date of the last medical assessment. Health Canada may conduct a fitness-to-work assessment if an employee is receiving DI or PSMIP-LTD benefits. Reassessment for a new medical certificate.
Canadian Panel Member Guide To Immigration Medical Examinations 2020 Canada Ca
Warhammer 40k Imperial Guard Medical Triage Warhammer 40k Imperial Guard Warhammer Warhammer 40k
Canadian Panel Member Guide To Immigration Medical Examinations 2020 Canada Ca
Class Ii Iv Medical Device Investigational Testing In Canada Vantage Biotrials
Canadian Panel Member Guide To Immigration Medical Examinations 2020 Canada Ca
Pin On Idiopathic Pulmonary Fibrosis
404 Not Found Nursing Assessment Emergency Nursing Nursing School
Pance Prep Pearls 2nd Edition Pdf Textbook Free Books Online Books To Read Online Download Books
Canadian Panel Member Guide To Immigration Medical Examinations 2020 Canada Ca
Canadian Panel Member Guide To Immigration Medical Examinations 2020 Canada Ca
Pin On All Academic Assignments Found Here
Overview Of The Medical Device Psur And Pmsr In The European Mdr
Littmann Classic Stethoscope Stainless Chestpiece Littmann Stethoscope Best Stethoscope Stethoscope
Clinical Trials Medical Device Trials Genesis Research Services
Canadian Panel Member Guide To Immigration Medical Examinations 2020 Canada Ca
Essay On Exam Malpractice In Nigeria In 2021 Essay Personal Statement Machine Learning
Class Ii Iv Medical Device Investigational Testing In Canada Vantage Biotrials




Posting Komentar untuk "Health Canada Category Iii Medical Assessment"