Health Canada Recall Policy
Health Canada recalls three batches of cannabis pre-rolls Products sold to licensed retailers in Yukon the Northwest Territories and Alberta as well as to patients via Medical Cannabis by Shoppers Drug Mart. The recall for Natural Balance Limited Ingredient Diets Green Pea Chicken Formula Dry Cat Food was.

Best Active Recall Strategies Nursing Motivation Nursing Mom Fashion Recall
Section C01051 of the Food and Drug Regulations requires a manufacturer or importer who commences a recall to submit information to Health Canada forthwith.

Health canada recall policy. Section C01051 of the Food and Drug Regulations requires a manufacturer or importer who commences a recall to. Recent recalls and alerts. Health Canada has posted a recall alert from Philips announcing that some of their ventilators and CPAP machines may pose a risk to patients because a foam used.
The responsible party should notify Health Canada if there is a potential need to recall a distributed product at the time a risk to health is identified. Health Canada has issued a recall that could harsh some Canadians cannabis buzz. Stop using this product immediately.
HOME-BOND and VIA-BOND Epoxy products recalled. It is an action taken by a manufacturer distributor or importer to carry out their responsibility to protect the public health. It will help facilitate understanding and compliance with sections of the Food and Drugs Act the Act the Food and Drug Regulations FDR and Natural Health Products Regulations NHPR that relate to recalls.
Here is the list of affected products according to Health Canadas website. Recalls and safety alerts are sent out when we have important information to sharemeaning you can feel more secure when choosing and using products. Recalls and Safety Alerts provides access to a comprehensive list of recalls advisories and safety alerts including recalls from Health Canada the Canadian Food Inspection Agency and Transport Canada.
At the following number 1-855-961-9420. The responsible party should notify Health Canada if there is a potential need to recall a distributed product at the time a risk to health is identified. Publisher - Current Organization Name.
Recall - Background and Objectives. Health Canada recommends that any individual affected by the recall immediately stop using the recalled product and to contact Organigram Inc. So far there are no reports of injuries related to the recall or the malfunctioning of the spray canisters.
Health Canada is recalling a brand of cat food after determining it may be contaminated with salmonella potentially causing both pets and people to fall ill. The agency is pulling three batches of pre-rolled joints that may be. If you are not able to notify us within this time frame you must provide a rationale in your initial recall report.
Cases of myocarditis andor pericarditis following immunization with COVID-19 vaccines. Health Canada says some canisters are not spraying anything when used and that could put you in a very dangerous situation when face to face with large mammals. All lots of Yummy Sports Candies BCAA powder all flavours recalled due to missing safety information for pregnant and breastfeeding women.
Recalls and safety alerts are sent out when we have important information to sharemeaning you can feel more secure when choosing and using products. This guide is for anyone working with drugs or natural health products. When a product is recalled or an advisory or alert is issued it means our surveillance tools are working.
Nestlé brand Drumstick Vanilla Chocolate Swirl Non-Dairy Frozen Dessert Cones recalled due to undeclared milk. When a product is recalled or an advisory or alert is issued it means our surveillance tools are working. Health Canadas Recall Policy further specifies that you must provide verbal or written notification to Health Canada within 24 hours of deciding to proceed with a recall.
Recall is an effective method of removing or correcting violative products that may represent a health hazard to the consumer or user. Recallposted for some ventilators due to possibility foam could be inhaled by patients Health Canadahas posted a recall alert from Philips announcing that some of their ventilators and CPAP machines may pose a risk to patients because a foam used in the products has been found to degrade and could potentially be inhaled into the lungs of patients. Health Canada has updated the product monographs labels for the Pfizer-BioNTech and Moderna COVID-19 vaccines to describe very rare reports of myocarditis inflammation of the heart muscle and pericarditis inflammation of the tissue surrounding the heart following vaccination.

Philips Respironics Positive Pressure Device Recall Canadian Sleep Society Css
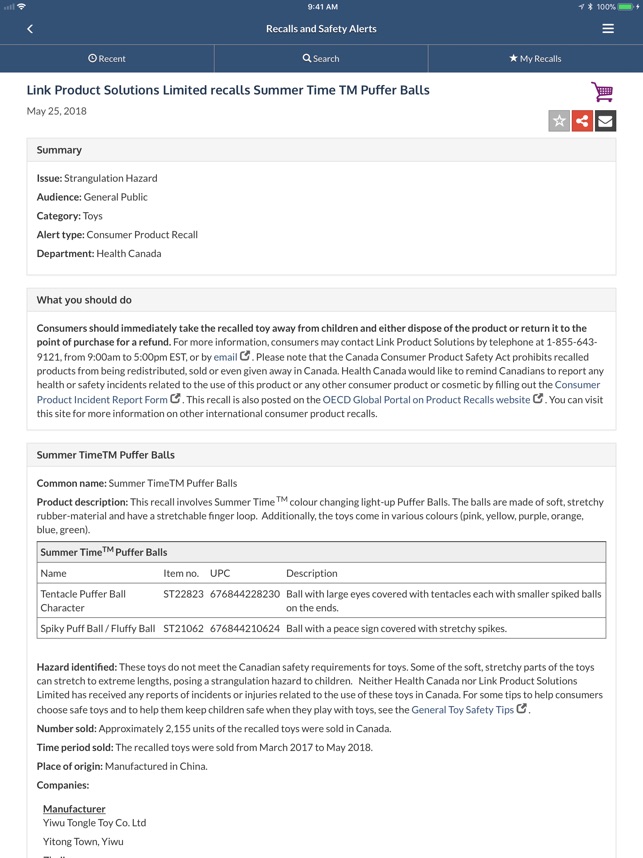
Recalls And Safety Alerts On The App Store

Bad Driving Habits Driving Habits Bad Drivers Driving

Health Canada Issues Advisory Over Masks Containing Graphene Urges Recall National Globalnews Ca

Product Recall Drivers And Trends

Product Recall Drivers And Trends

Product Recall Procedure Template Guidance Free Download
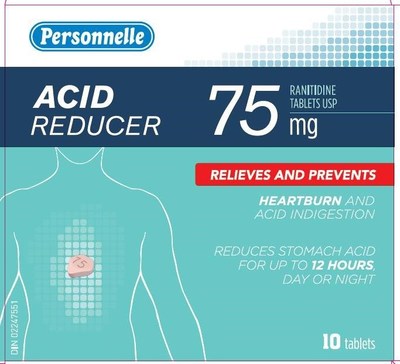
Information Update Ranitidine Products Recalled Because Of A Nitrosamine Impurity

Information Update Ranitidine Products Recalled Because Of A Nitrosamine Impurity

Notifications Of Official Enforcement Actions Fsis Has Taken Against Establishments That Have Been Found In Violation Of The H Food Safety Dog Food Recall Food

Avocado Recall In 6 States Over Listeria Concerns Cnn Recall Avocado Fast Facts
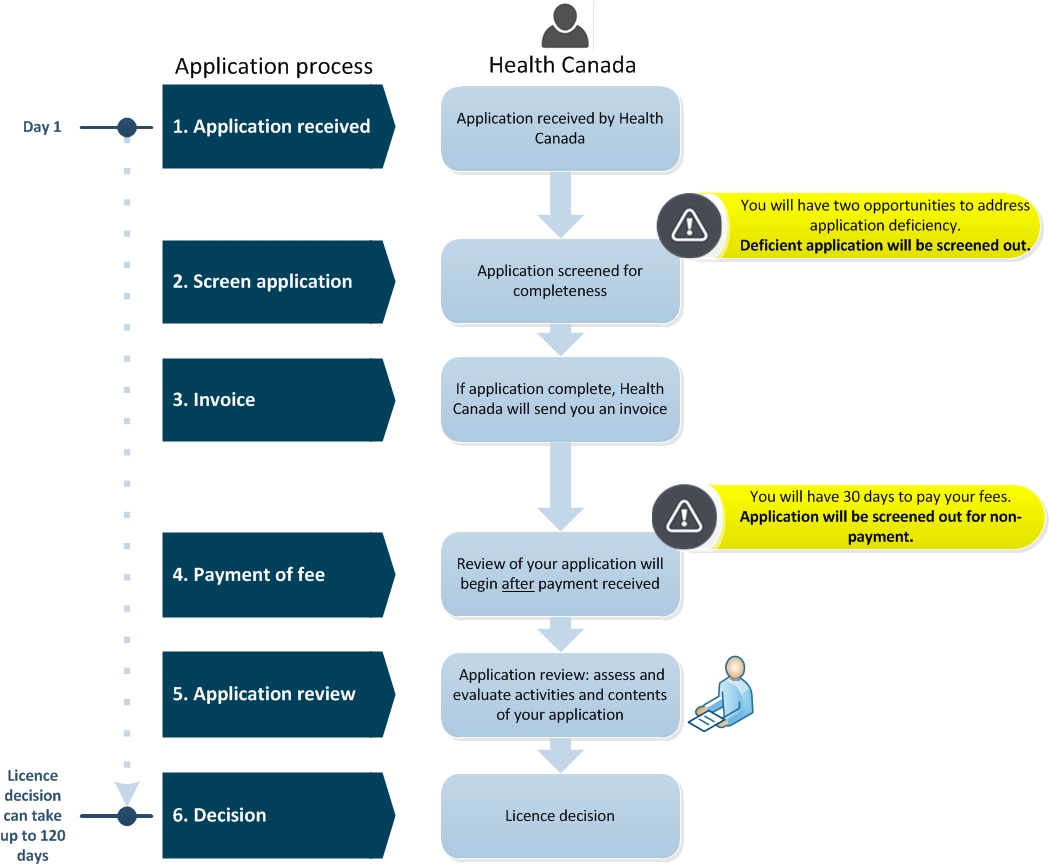
Frequently Asked Questions Medical Device Establishment Licensing And Fees Canada Ca

Template Ideas Cleaning Schedule For Office Open Scheduleate Within Menu Checklist T Cleaning Checklist Template Cleaning Schedule Templates Checklist Template

Product Recall Drivers And Trends

Tumblr Est Un Lieu Ou Vous Pouvez Vous Exprimer Apprendre A Vous Connaitre Et Creer Des Liens Autour De Vos Centres D Interets Amado Social Work Total Recall

Recall Policy For Health Products Canada Ca



Posting Komentar untuk "Health Canada Recall Policy"