Health Canada Adverse Reaction Database
Data extracts from the Canada Vigilance adverse reaction online database. Query page of Health Canadas Canada Vigilance Online Database which contains reports of suspected adverse reactions submitted by health professionals Market Authorization Holders and consumers since 1965.
They include anaphylaxis a severe allergic reaction which has been reported 69 times for all COVID-19 vaccines across Canada.

Health canada adverse reaction database. Health Canada has made its database of adverse drug reactions available to Canadians in a searchable online format Health Minister Ujjal Dosanjh announced in a release on Wednesday. However some adverse reactions or problems may become evident only after a product is in use by consumers. The information on this page reflects detailed case information data from the Public Health Agency of Canadas Canadian Adverse Events Following Immunization Surveillance System CAEFISS and Health Canadas Canada Vigilance program.
Health Canadas Drug Identification Numbers DIN are assigned to drug products that are authorized for sale in Canada and are listed on the labels of prescriptions and over-the-counter drug products. ARs for marketed health products within the scope of this guidance document are to be reported to the Canada. Your report helps to indicate if there is an issue with a health or cannabis product a signal.
The Canada Vigilance Program collects adverse reaction reports for the following marketed health products approved for use in humans. These reports included reactions to. Report an adverse reaction or side effect.
However Health Canada is aware that adverse reactions are often under-reported to both voluntary and mandatory spontaneous surveillance systems. Consumers and health professionals who submit reports voluntarily. For industry information about COVID-19 visit our COVID-19 Drugs and vaccines section.
Learn and better understand the importance of reporting side effects. MedEffect Canadas Adverse Reaction Online Database contains information on suspected adverse reaction reports related to marketed health products that were submitted to Health Canada by consumers and health professionals who submit reports voluntarily as well as by Market Authorization Holders manufacturers and distributors who are required to submit reports according to the Next link will. MedEffect Canada provides consumers patients and health professionals with easy access to.
Adverse reaction reports are submitted by. All marketed drugs and health products have benefits and risks. Obtain new safety information on drugs and other health products.
The data may undergo changes as more information about cases becomes available. The Canada Vigilance Adverse Reaction Online Database contains information about suspected adverse reactions also known as side effects to health products captured from adverse reaction reports submitted to Health Canada by consumers and health professionals who submit reports voluntarily as well as by market authorization holders manufacturers and distributors who are required to submit. 43 rows Although the Canada Vigilance Adverse Reaction Online Database is a relational.
The data extract is a series of compressed ASCII text files of the full data set contained in the Canada Vigilance Adverse Reaction Online Database. For industry information about COVID-19 visit our COVID-19 health product industry section. This guidance document provides market authorization holders MAHs with assistance on how to comply with the Food and Drugs Act the Food and Drug Regulations and the Natural Health Products Regulations with respect to reporting adverse reactions ARs to marketed health products but excluding blood and blood components and cells tissues and organs.
The Canada Vigilance Adverse Reaction Online Database contains information on suspected adverse reaction reports related to marketed health products that were submitted to Health Canada by consumers and health professionals who submit reports voluntarily as well as by Market Authorization Holders manufacturers and distributors who are required to submit reports according to the Food and Drugs. The number of adverse reports in the Canada Vigilance Adverse Reaction Online Database should not be used as a basis for determining the incidence of a reaction or for estimating risk of a particular product as neither the total number of. Two Databases From 19651987 physicians pharmacists public health nurses hospitals manufacturers and consumers reported adverse reactions to Health Canada for inclusion in the Canada Vigilance CV on-line database.
The Canada Vigilance Adverse Reaction Online Database contains information about suspected adverse reactions also known as side effects to health products. Health Canada Drug Product Database DPD 15000 DINs. A subset of de-identified Canada Vigilance adverse reaction reporting program data is made publicly available from the Canada Vigilance adverse reaction online database.
All health products are carefully evaluated before they are licensed in Canada. Most adverse events are mild and include soreness at the site of injection or a slight fever. Pharmaceutical drugs prescription and non-prescription Biologics Schedule D biotechnology products therapeutic and diagnostic vaccines and fractionated blood products Radiopharmaceuticals drugs Natural health products Cells Tissues and Organs.
Trend and safety data in a de-identified format may be communicated by a variety of risk communication tools andor responses to inquiries. Drugs 8 days ago The database covers approximately 15000 drug products. ZIP Version - 202966 MB The data set is updated on a monthly basis and currently covers the following time period.
Serious adverse events are rare but do occur.

Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca
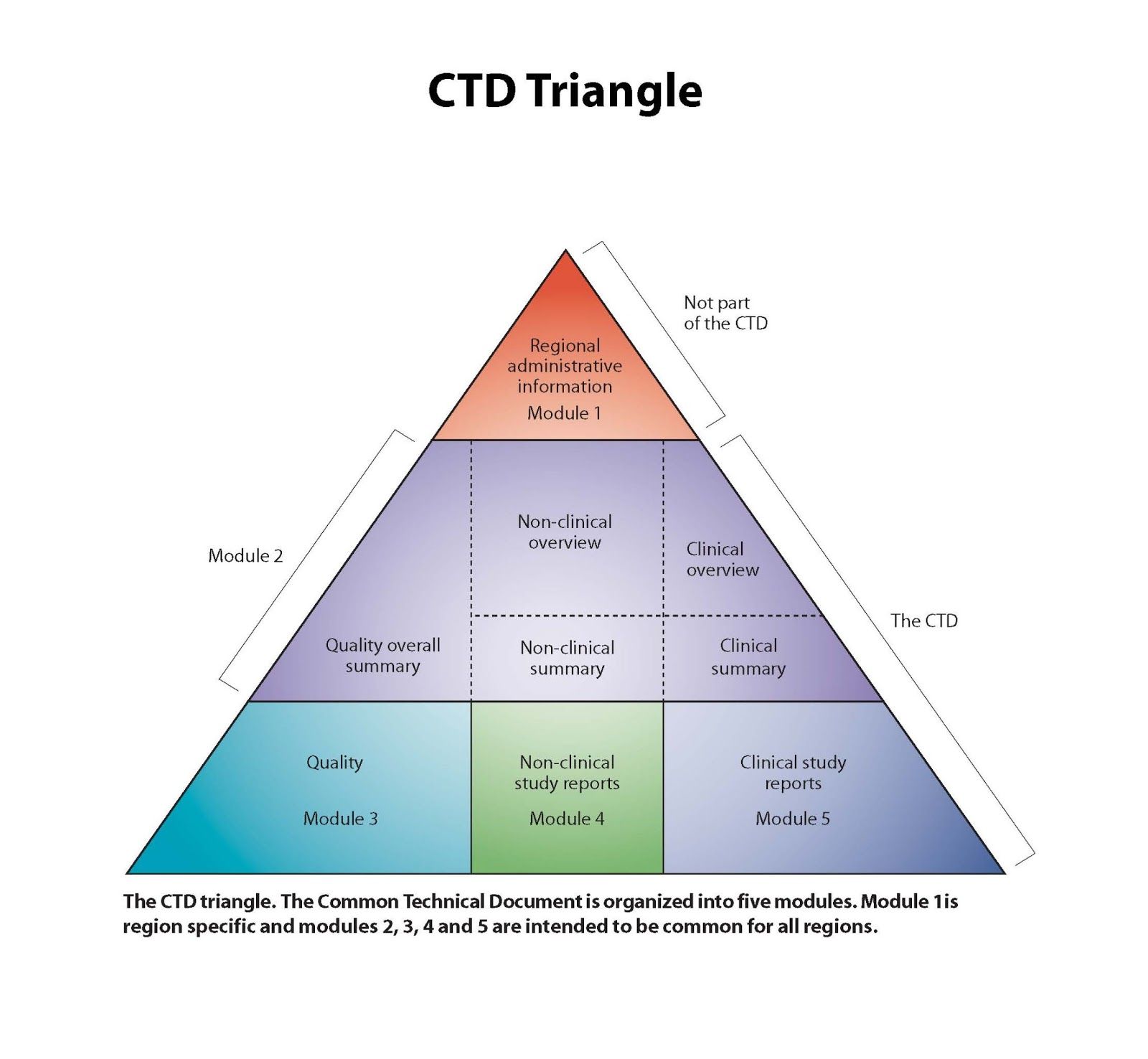
The Drug Review And Approval Process In Canada An Eguide Spharm Canada S Drug Regulatory Experts

Fig 3 World Health Population Transforming Health Workers Education For Universal Health Coverage Global Challenges Health Workers Challenges Education

Natural Alternatives To Cortisone Prednisone Vitality Magazine Toronto Canada Alternative Health Natural Alternative Natural Medicine Alternative Health
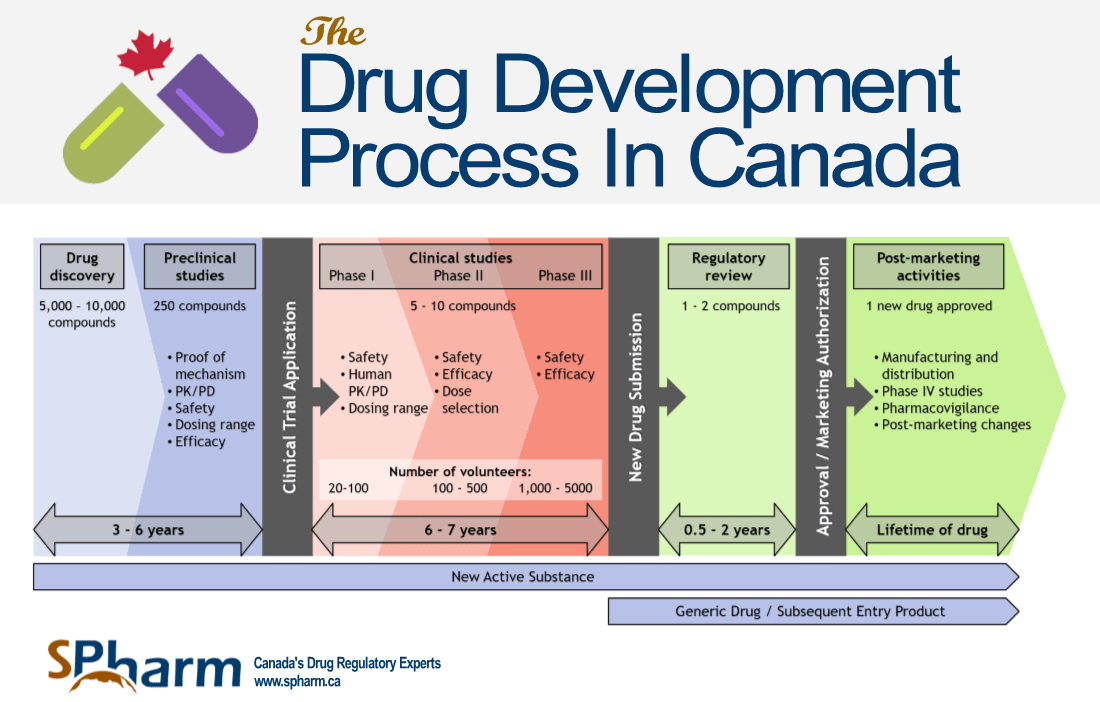
The Drug Review And Approval Process In Canada An Eguide Spharm Canada S Drug Regulatory Experts

Mandatory Reporting Of Serious Adverse Drug Reactions And Medical Device Incidents By Hospitals Guidance Document Canada Ca

Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca

Reporting Adverse Reactions To Marketed Health Products Guidance Document For Industry Canada Ca

Building And Sustaining A Culture Of Innovation In An Academic Health Centre In 2020 Charts And Graphs Innovation Strategic Planning

Comparison Of Regulatory Frameworks Of Environmental Risk Assessments For Human Pharmaceuticals In Eu Usa And Canada Sciencedirect
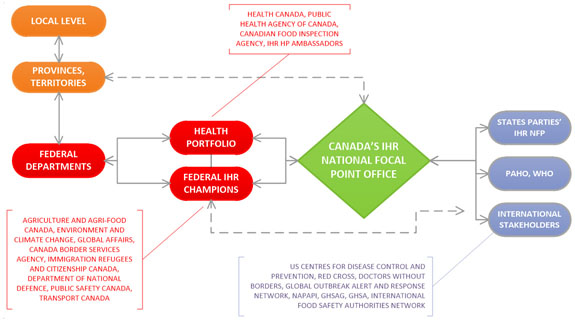
International Health Regulations Joint External Evaluation Of Canada Self Assessment Report Canada Ca
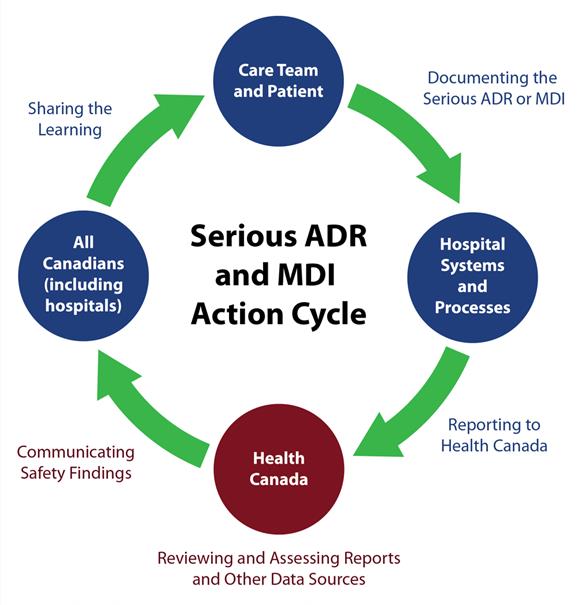
Module 1 Overview Of Vanessa S Law And Mandatory Hospital Reporting Requirements Canada Ca

Cadth Canadian Drug Expert Committee Recommendation Bictegravir Emtricitabine Tenofovir Alafenamide Biktarvy Gilead Sciences Canada Inc Ncbi Bookshelf

Health Product Infowatch October 2018 Canada Ca
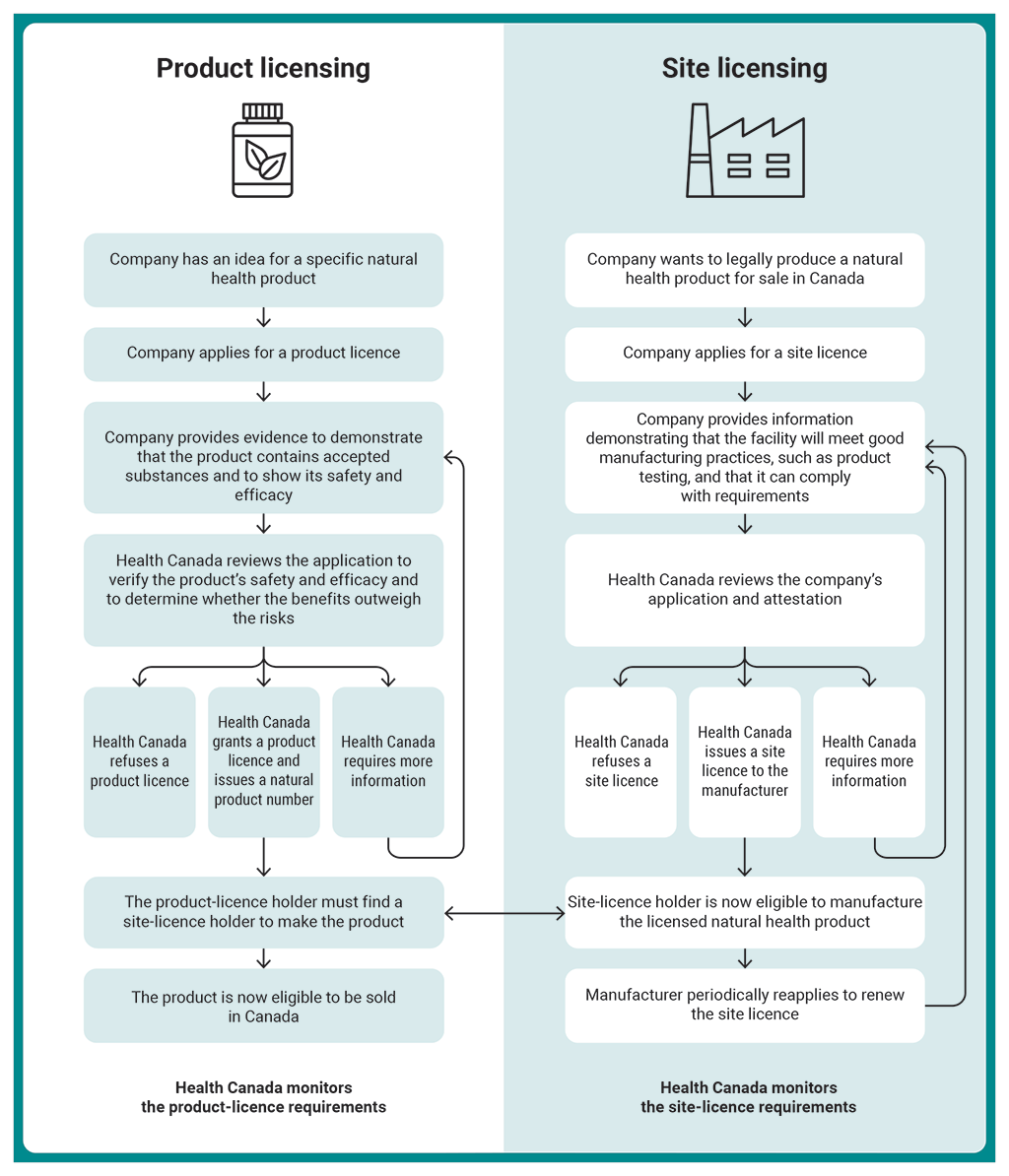
Report 2 Natural Health Products Health Canada

Pin Em Btc Health Bloggers Board

Health Product Infowatch June 2020 Canada Ca
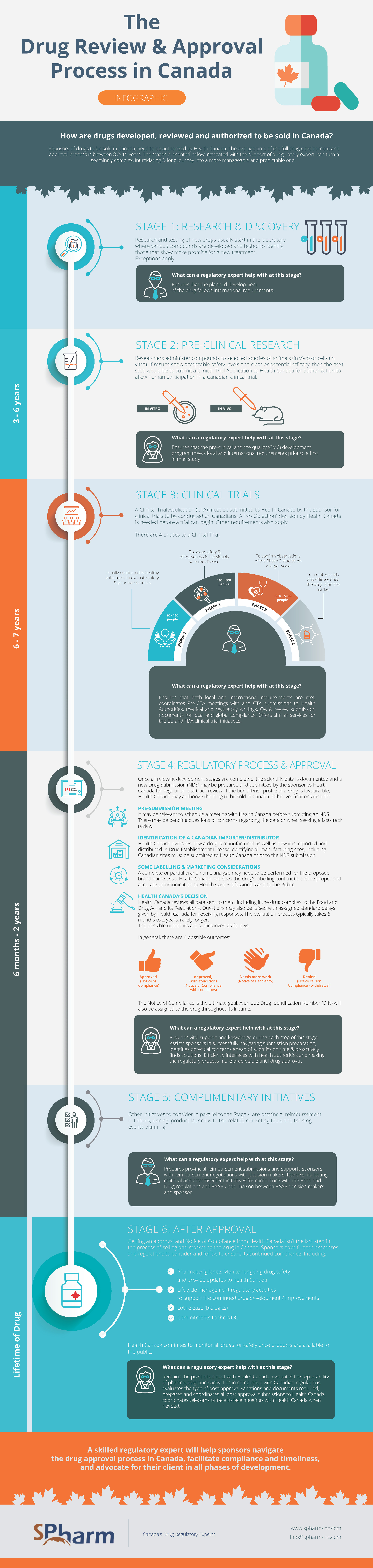
The Drug Review And Approval Process In Canada An Eguide Spharm Canada S Drug Regulatory Experts

Posting Komentar untuk "Health Canada Adverse Reaction Database"