Health Canada Monograph Database
Search the Drug Product Database DPD to find drugs authorized for sale by Health Canada. Note - The product information found within this database originates from organizations not subject to the Official Languages Act and is available in the language in which it was written and submitted to Health Canada.
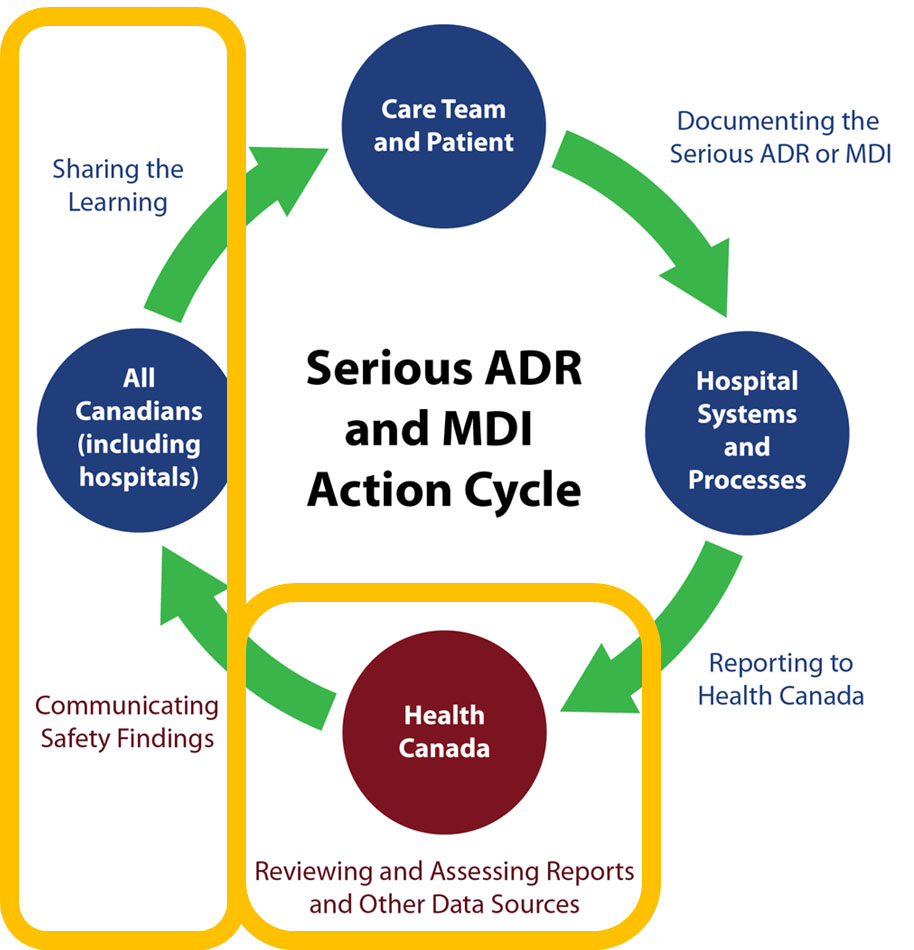
Module 4 Health Canada S Review And Communication Of Safety Findings Canada Ca
The DPD is updated nightly and includes.

Health canada monograph database. Health canada database containing product specific information on drugs approved for use in canada. Well being Is The Most Essential Wealth - In case youre lucky enough to have employer-provided medical insurance that narrows your choices down to the plans that your employer affords. Simple search Simple Search allows only one search field criterion be specified where Advanced Search allows multiple.
Ingredients must be chosen from the current Natural Health Products Ingredients Database18 and must meet the limitations outlined in that database the Food and Drugs Regulations FDR 19 the Herbs used as Non-medicinal Ingredients in. HEALTH PROFESSIONAL INFORMATION 1 INDICATIONS. Health Canada Product Monograph Database.
Health Canada Product Monograph Database. Sunscreen Monograph 2 FOREWORD Health Canada is pleased to announce the release of the final Sunburn Protectants Monograph. The veterinary labelling is developed by the drug sponsor according to the Food and Drug Regulations.
Therefore Health Canada has not authorized an indication for pediatric use. A monograph selection is required for compendial applications where the entire product. The product monograph is developed by a drug sponsor according to guidelines published by Health Canada that provide direction on the content and format.
Due to the fact that the information originated with an organization that is not subject to the Official Languages Act the document may only appear in the language in which it was written. In addition the Patient Medication Information and Patient Information Card can be found inside the MIFEGYMISO box. A monograph is a written description of particular elements on an identified topic.
Translations of the document are the responsibility of the sponsor involved. Drug Product Database online query. The Web application provides an interface to the ingredient terminology used in the Natural Health Products Ingredients Database NHPID to Natural Health Products Directorate NHPD single-ingredient monographs and Abbreviated Labelling Standards AbLS.
29 when i search for product monographs using the drug product database why are some available in english and french and others only available in english. The Compendium of Monographs can help speed the evaluation of the safety and efficacy of medicinal ingredients commonly used in natural health products. If you do not have coverage by your job perhaps an organization or association that you simply belong to will assist you to buy medical insurance through them at a.
VASCEPA Product Monograph Page 3 of 19 PART I. Of the total number of patients in well-controlled clinical studies of VASCEPA 45 were 65 years of age and. Availability of the drug in Canada product monograph.
The product monograph can also be accessed on the Health Canada website. 614 Geriatrics Geriatrics 65 years.

The Herbarium Membership Resource For Herbalists Plant Monograph Database In Depth Articles Videos And More Herbal Academy Herbalism Herbalist

The South Asian Health Foundation Uk Guidelines For Managing Diabetes During Ramadan Diabetes Research And Clinical Practice

Drug And Natural Health Products Recall Guide Canada Ca

The Lost Book Of Remedies Does It Really Work In 2021 Herbalism Herbal Remedies Herbal Medicine

After Agnes Martin Untitled Agnes Martin Art Rosenquist
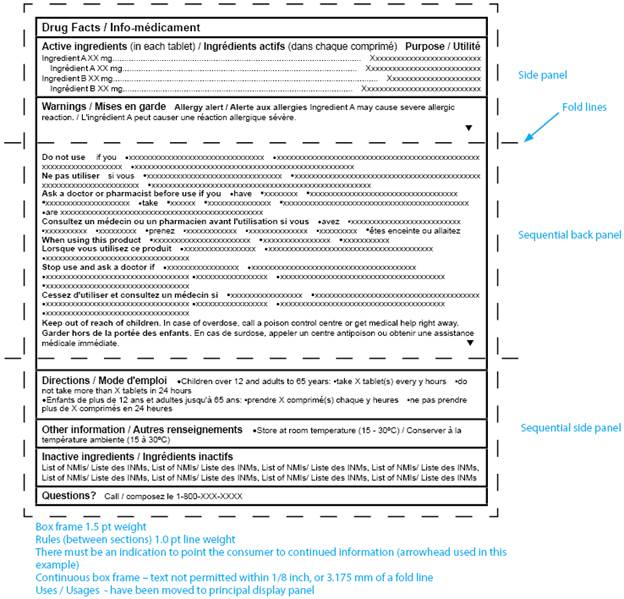
Labelling Requirements For Non Prescription Drugs Guidance Document Canada Ca

Health Product Infowatch July 2019 Canada Ca
Http Pi Lilly Com Ca Bamlanivimab Ca Pm Pdf

Health Canada And Drug Product Information Xml Product Monographs Format

Good Label And Package Practices Guide For Non Prescription Drugs And Natural Health Products Canada Ca

Health Product Infowatch June 2020 Canada Ca

Health Product Infowatch November 2020 Canada Ca

Reporting Adverse Reactions To Marketed Health Products Guidance Document For Industry Canada Ca

Operation Room Workflow Operation Room Scrub Tech Phlebotomy Scrubs

Mandatory Reporting Of Serious Adverse Drug Reactions And Medical Device Incidents By Hospitals Guidance Document Canada Ca

Comprehensive Review In Clinical Neurology A Multiple Choice Book For The Wards And Boards Secon Ebook Neurology Books To Read Online

Health Canada Guidance On Anonymization And Redaction Of Clinical Data

Posting Komentar untuk "Health Canada Monograph Database"