Office Of Clinical Trials Health Canada
A Clinical Trial Application CTA must be filed with Health Canada prior to the initiation of a clinical trial in Canada. Associated Director Office of Clinical Trials TPD.

Pin By Vuvu On Programmes Office Health Care Pharma Pharmaceutical Company
Health Canada Review A Clinical Trial Application CTA must be filed with Health Canada prior to the initiation of a clinical trial in Canada.

Office of clinical trials health canada. Clinical Trials Handbook Canada Baker McKenzie. Health Canada is pleased to announce the release of the finalized Guidance Document for Clinical Trial Sponsors. 3 The sponsor may be an individual corporate body institution or organization that submits the CTAP and is ultimately responsible for the conduct of a CT.
The database is managed by Health Canada and provides a source of information about Canadian clinical trials involving human pharmaceutical and biological drugs. 3105A Ottawa Ontario Canada K1A 0K9 Phone. The Office of Clinical Trials is looking for an individual with either a Masters degree or PhD who has experience reviewing analyzing and Posted by Carole Legare Aujourdhui en cette Journée.
Health Canada through its Clinical Trials Database is providing to the public a listing of specific information relating to phase I II and III clinical trials in patients. A protocol which details the objectives benefits risks methods and conditions for the trial to function Clinical Trial Site Information Form. Office of Clinical Trials Therapeutic Products Directorate Health Products and Food Branch Health Canada Address Locator.
Clinical trial search. Canada October 10 2018 On July 9 2018 the Federal Court released its decision ordering Health Canada to provide the results of certain clinical trials including participant level datasets to an. You may search by one or more of the criteria immediately below or alternatively by either Protocol Number or Control Number.
An overview will be provided of Health Canadas Clinical Trial Compliance Program including how the program continued providing oversight during the pandemic the plan for this fiscal year and program updates. Clinical Trial Applications which provides guidance to all sponsors for example eg industry academic contract research organization seeking authorization to sell or import a drug for the purpose of a clinical trial in Canada. The position title is Medical Evaluator Office of Clinical Trials Medical Group.
When typing inside fields do not include punctuation marks such as hyphens commas colons brackets and wildcard characters. The sponsor must notify Health Canada of any changes to the. The Health Canada application includes.
In this light Health Canada has authorized a nation-wide clinical trial CONCOR-1 study on the use of convalescent plasma to treat COVID-19. Uncategorized office of clinical trials health canada. The legal requirements for NHP clinical trials are found in the NHP Regulations which are administered by the Natural Health Products Directorate NHPD.
A clinical trial is the best way to obtain the necessary information systematically to determine whether convalescent plasma is a. Office of Clinical Trials Therapeutic Products Directorate 5 th Floor Holland Cross Tower B AL 3105A 1600 Scott Street Ottawa Ontario Canada K1A 0K9 Tel. 145 - 230 Clinical Trials Regulatory Modernization Carole Legare MD Director Office of Clinical Trials Health Canada.
Pauline Kerr is employed with Health Canada registered with Shared Services Canada.

Biotrial Your Partner In Non Clinical Research And Clinical Trials Biotrial

Clinical Trial Infographics Google Search Clinical Trials Cancer Patients Clinic
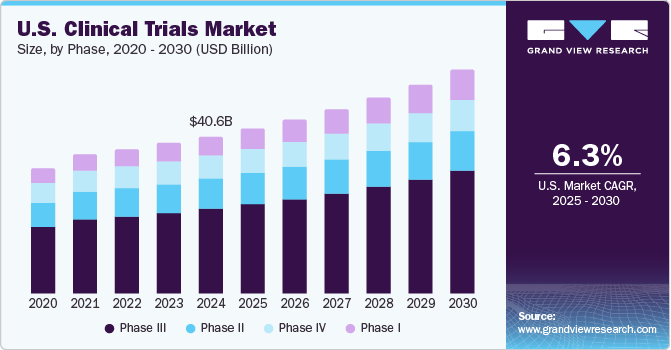
Clinical Trials Market Size Share Growth Report 2021 2028
How To Report Clinical Trial Results Research Ethics Compliance

Clinical Trials And Studies Iffgd
Quality By Design For Clinical Trials Socra
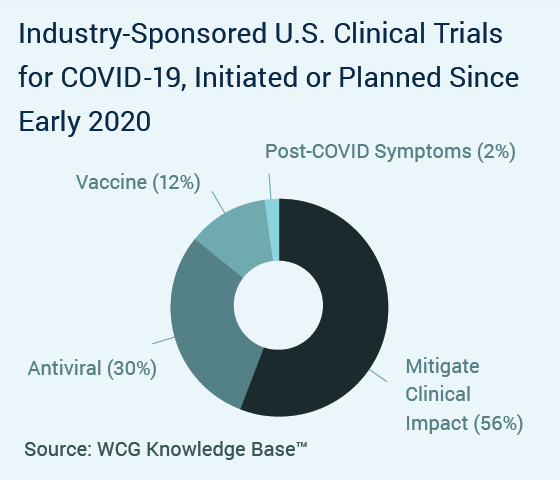
Clinical Trial Insights From The Wcg Knowledge Base Wcg Clinical

Clinical Trials And Studies Iffgd
Clinical Trial Solutions Iqvia

Dr Whitley Fda On Clinical Trials Clinical Trials Clinic Doctor

Clinical Trials And Studies Iffgd
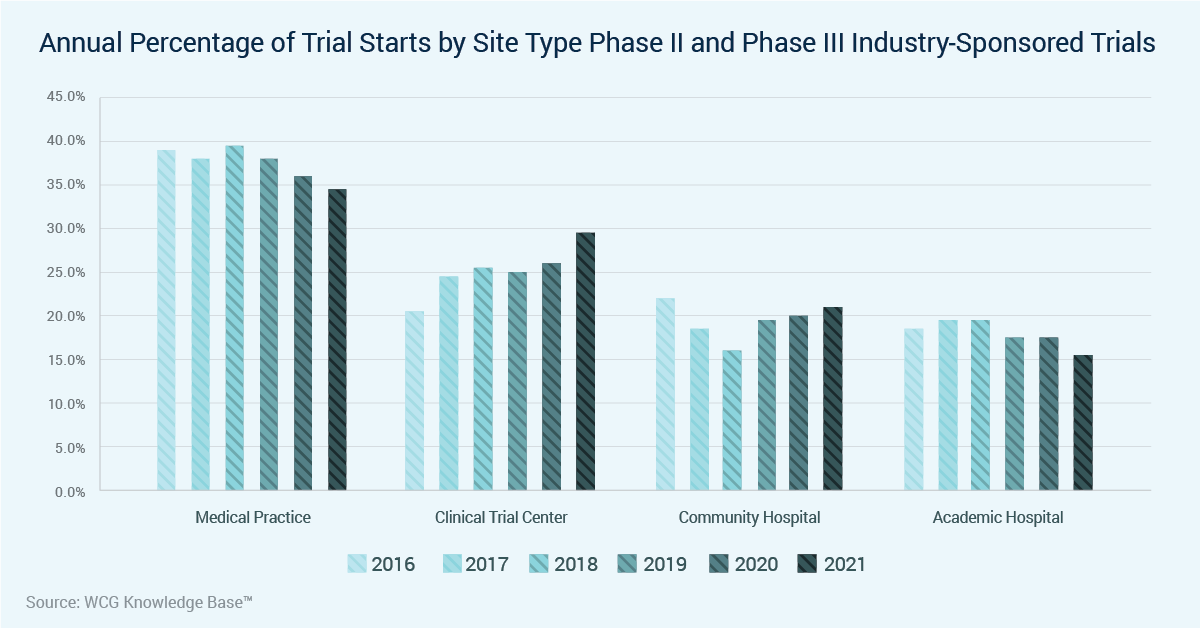
Clinical Trial Insights From The Wcg Knowledge Base Wcg Clinical

Boehringer Ingelheim S Creative Use Of Infographics For Patient Recruitment In Clinical Trials Clinical Trials Clinical Research Boehringer Ingelheim






Posting Komentar untuk "Office Of Clinical Trials Health Canada"