Clinical Trial Database Health Canada
Health Canada has hosted a Clinical Trials Database for drugs since 2013 and encouraged the clinical trial registration in international registries for drugs and devices for a number of years. It is important to note that before a clinical trial can start it.
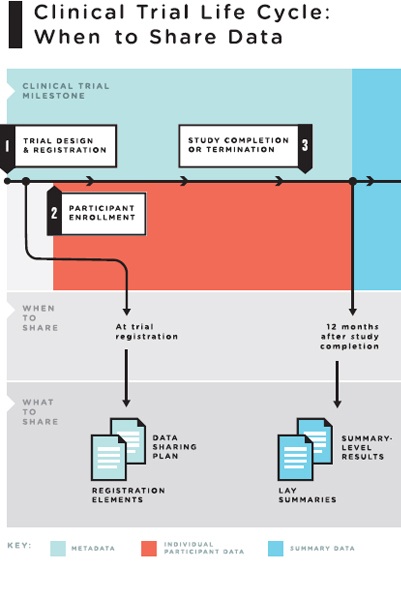
4 The Clinical Trial Life Cycle And When To Share Data Sharing Clinical Trial Data Maximizing Benefits Minimizing Risk The National Academies Press
Although Health Canada has more information including sites where patients are enrolled it will inlcude only limited information.
Clinical trial database health canada. Gives you access to information about each type of drug and health product inspection done by Health Canada in Canada and abroad covers all inspections done since 2012 in Canada and abroad tells you what health product establishments are licensed or registered by Health Canada replaces the drug establishment licence live listing. Companies that make drugs or medical devices send this information to Health Canada to help it review products for sale in Canada. The database is managed by Health Canada and provides a source of information about Canadian clinical trials involving human pharmaceutical and biological drugs.
Health Canada reviews the documents submitted in CTAs and CTA-As to assess the quality of the products and determine that the use of the drug for the purposes of the clinical trial does not endanger the health of clinical trial subjects or other persons the clinical trial is not contrary to the best interests of a clinical trial subject and the objectives of the clinical trial may be achieved. Health Canada requires researchers to sign a standard confidentiality agreement in order to release clinical trial data for the purpose of research. The database is managed by Health Canada and provides a source of information about Canadian clinical trials involving human pharmaceutical and biological drugs.
The health authority of Canada. However this does not indicate that the trial has started. Launched in May the database includes the titles of trial protocols the medical conditions involved the drugs and populations being studied enrolment status and the dates Health Canada authorized the trials.
On the basis of the researchers refusal to sign. These reports include information on the design and results of clinical trials. Clinical information is submitted in clinical study reports.
Ad Expertise In The Diabetes Clinical Trials. The Medicines and Healthcare products Regulatory Agency MHRA and Health Canada have published guidance to improve the safety of patients in clinical trials. The date when Health Canada issued a No Objection Letter to the trial sponsor which signifies that Health Canada considers the clinical trial acceptable.
Overview As per the CanadaFDA the CanadaFDR the G-CanadaCTApps and CAN-29 Health Canada HC is the competent authority responsible for clinical trial approvals oversight and inspections in Canada. 4 rows Canada does not have a clinical trial registry of its own. Clinical information is data about the safety and efficacy of drugs or medical devices used in humans.
The Clinical Trials Database provides to the public a listing of specific information relating to phase I II and III clinical trials in patients. When typing inside fields do not include punctuation marks such as hyphens commas colons brackets and wildcard characters. If a NHPMF or a Drug Master File DMF is filed with Health Canada and cross-referenced for certain proprietary information eg section 23S 22 of QOS-NHP template provide the NHPMF number or the DMF number assigned by Health Canada and a copy of the letter of access from the manufacturer allowing the NHPMF to be used in support of the clinical trial submission.
Additional information on Health Canadas CTD is available at. While compliance with this voluntary registration has been quite high it may not provide a complete picture of all trials taking place recruiting completed or discontinued. Health Canadas Clinical Trials Database is a listing of information about phase I II and III clinical trials in patients.
Clinical trial search. You may search by one or more of the criteria immediately below or alternatively by either Protocol Number or Control Number.
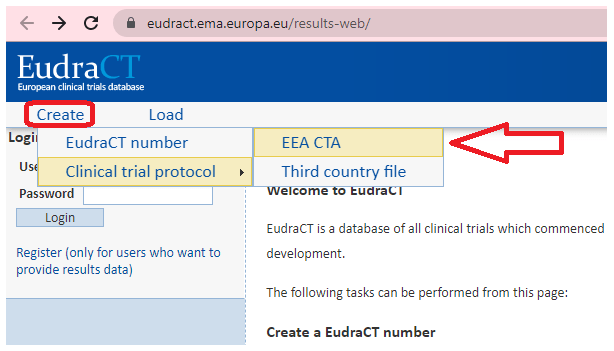
What Are The Documents Required For Clinical Trial Applications To Regulatory Authorities In Europe Sofpromed

Reporting Guidelines For Clinical Trial Reports For Interventions Involving Artificial Intelligence The Consort Ai Extension The Lancet Digital Health

A Real Time Dashboard Of Clinical Trials For Covid 19 The Lancet Digital Health

Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca
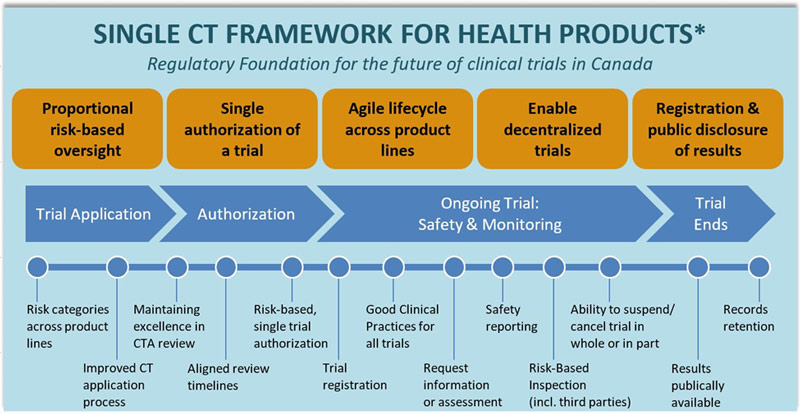
Clinical Trials Modernization Consultation Paper Canada Ca
Nih Clinregs Database Provides International Clinical Trial Regulations Fogarty International Center Nih

Ictrp Search Portal Advanced Search
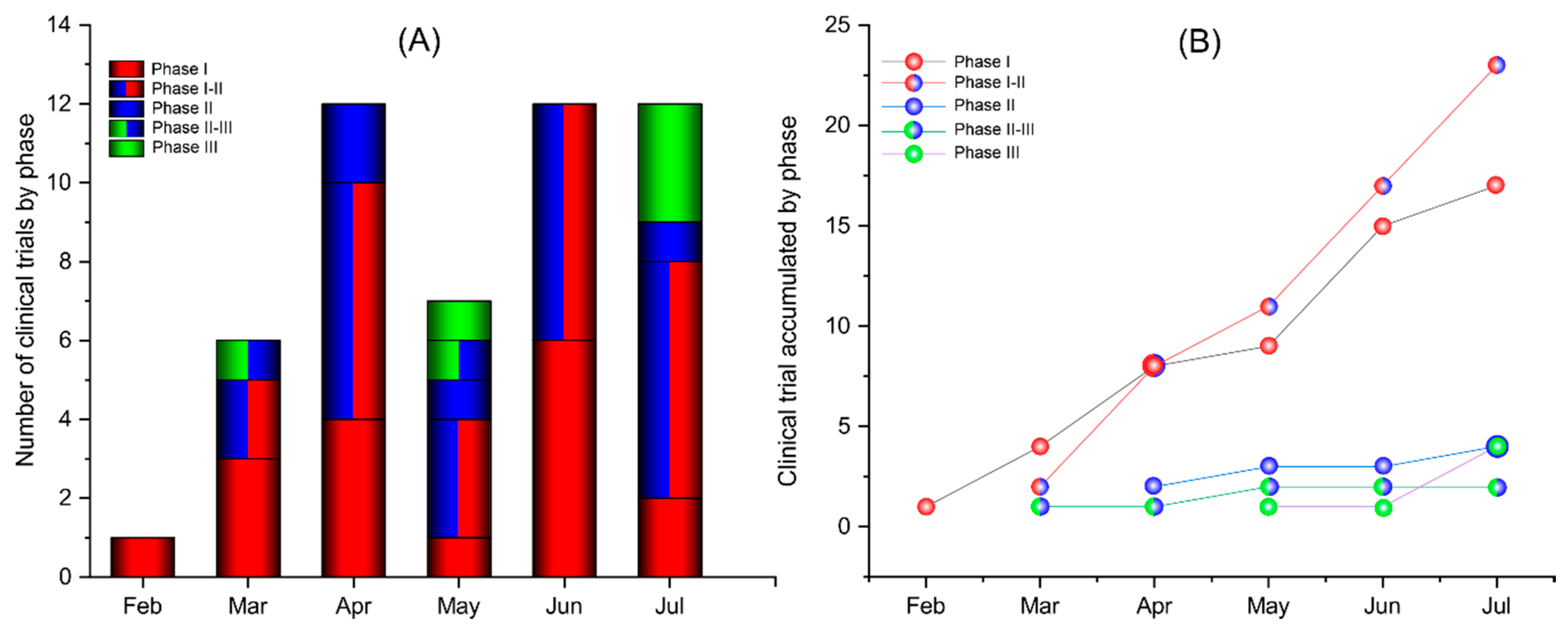
Vaccines Free Full Text Current Clinical Trials Protocols And The Global Effort For Immunization Against Sars Cov 2 Html
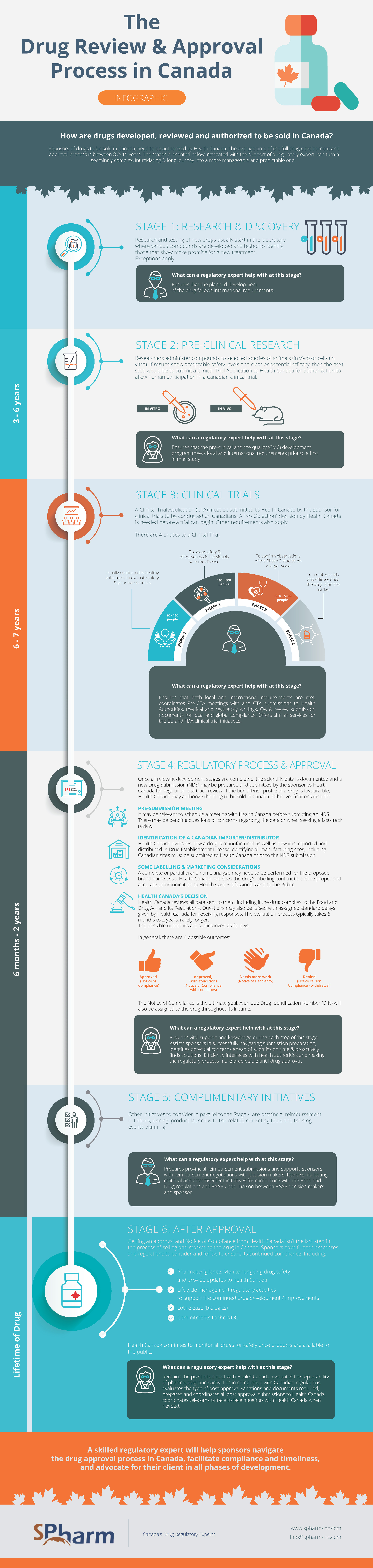
The Drug Review And Approval Process In Canada An Eguide Spharm Canada S Drug Regulatory Experts

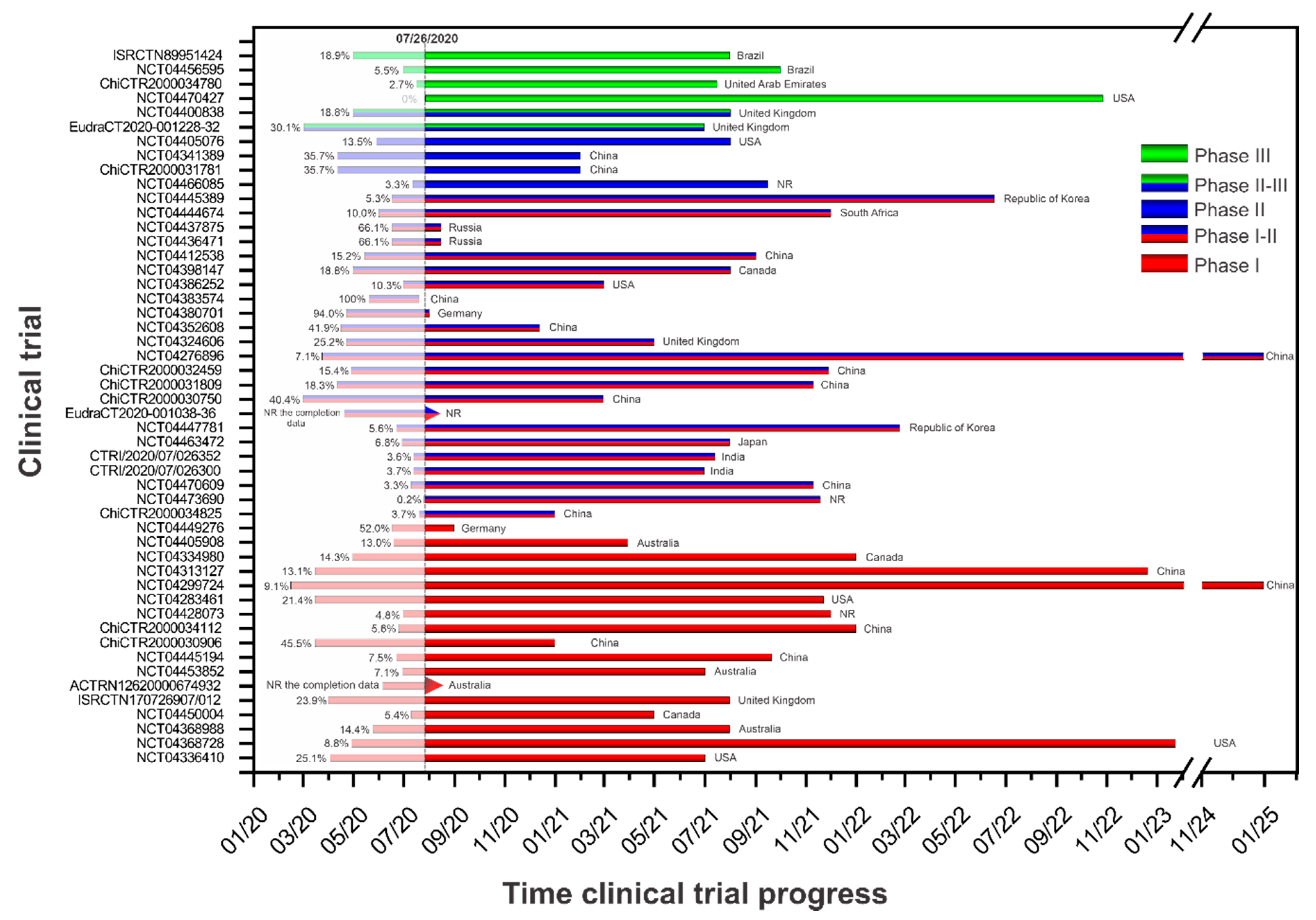
Review Of Trials Currently Testing Treatment And Prevention Of Covid 19 Clinical Microbiology And Infection

Vaccines Free Full Text Current Clinical Trials Protocols And The Global Effort For Immunization Against Sars Cov 2 Html
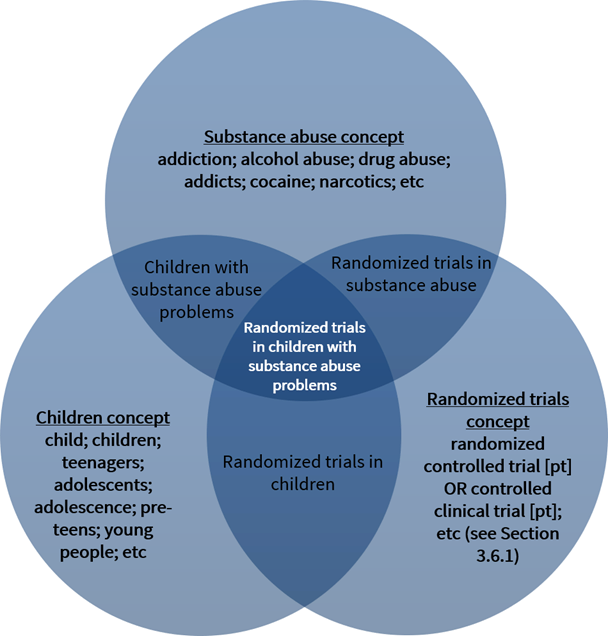
4 S1 Technical Supplement To Chapter 4 Searching For And Selecting Studies Cochrane Training
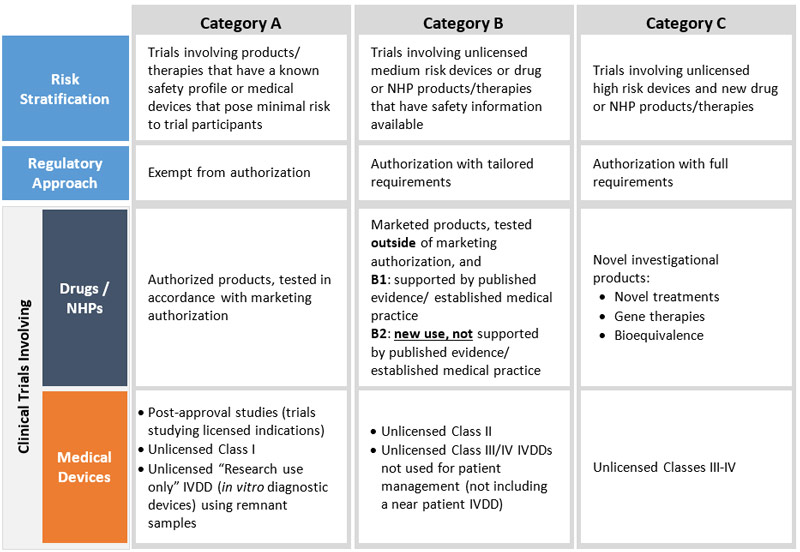
Clinical Trials Modernization Consultation Paper Canada Ca
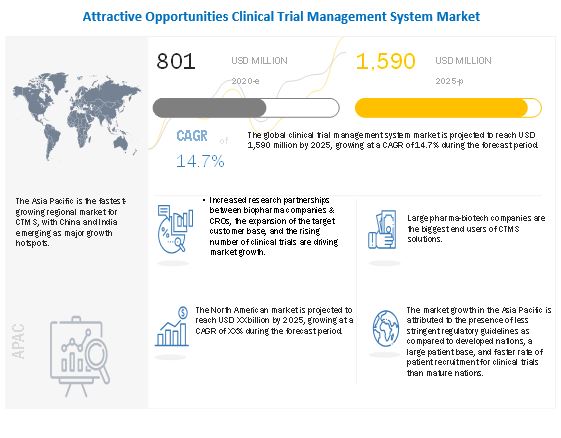
Clinical Trial Management System Market Global Forecast To 2025 Marketsandmarkets

An Introduction To Integrated Summary Of Safety And Integrated Summary Of Effectiveness Iss And Ise Quantics Biostatistics

Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca
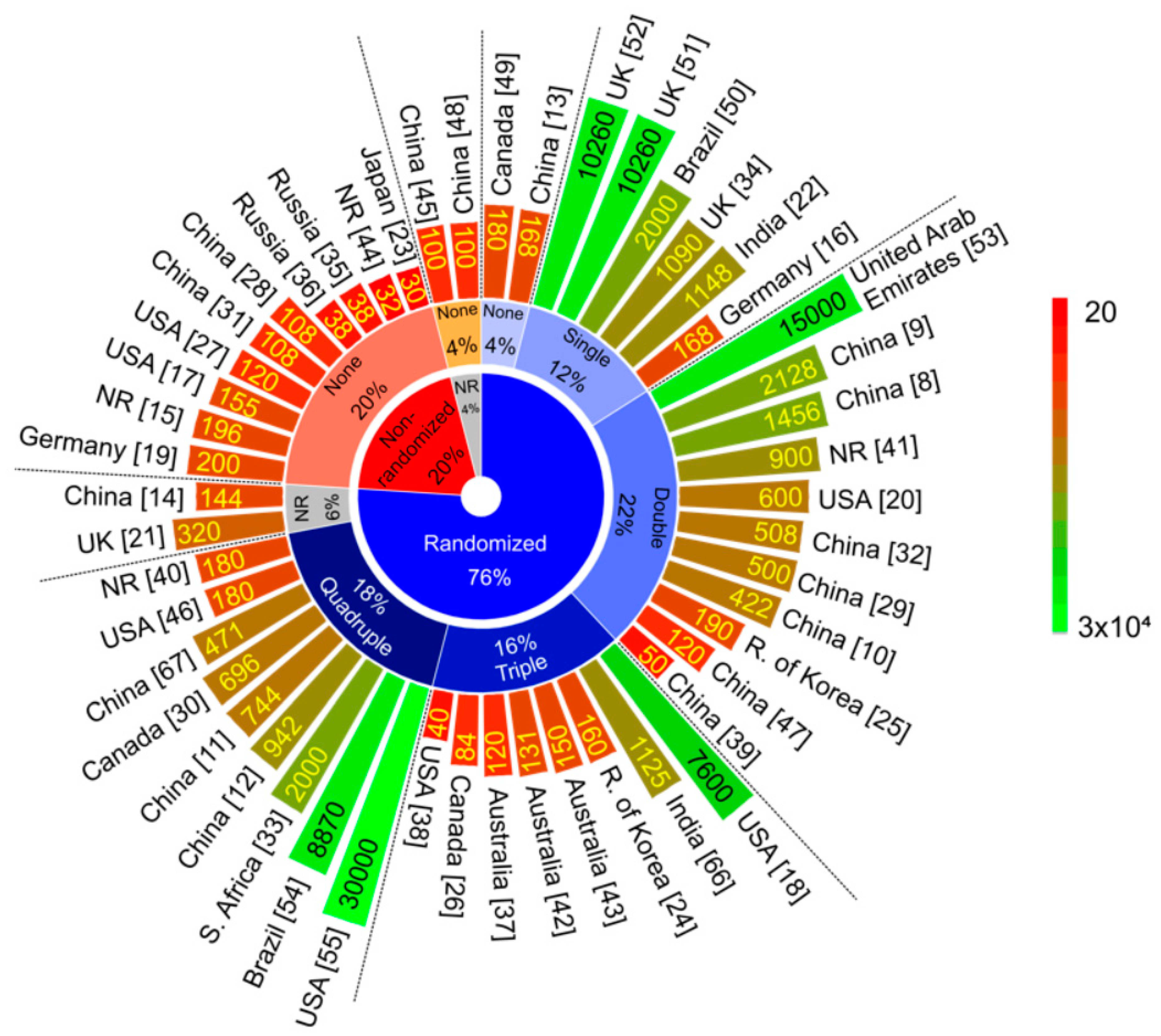
Vaccines Free Full Text Current Clinical Trials Protocols And The Global Effort For Immunization Against Sars Cov 2 Html
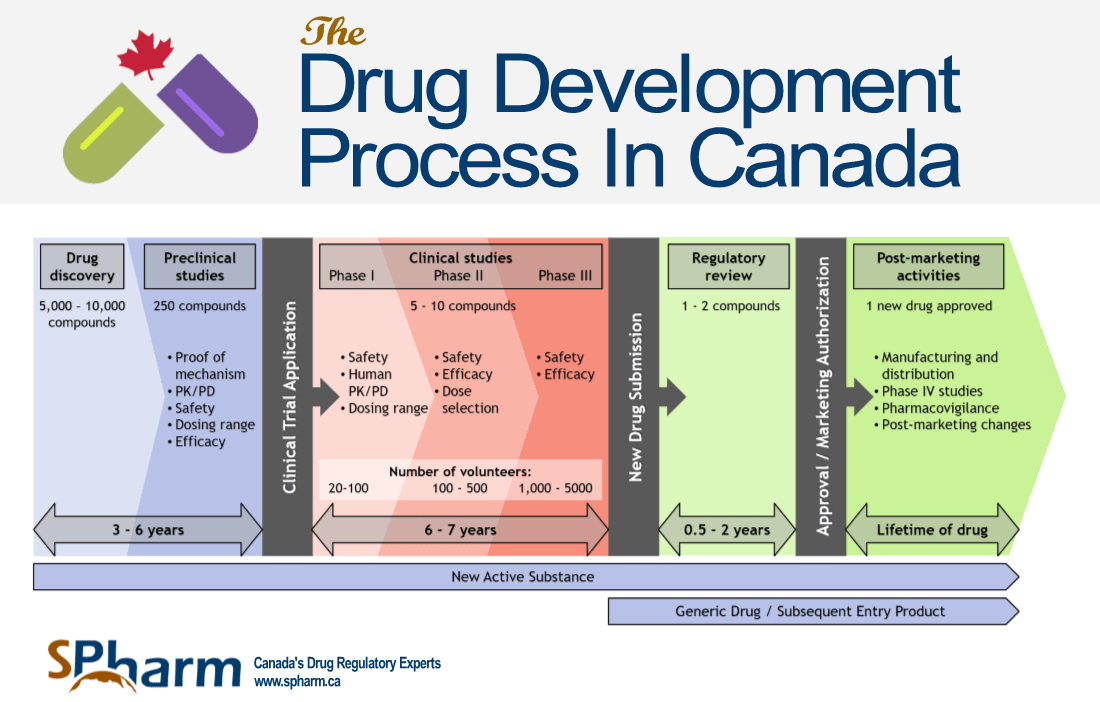
The Drug Review And Approval Process In Canada An Eguide Spharm Canada S Drug Regulatory Experts

Posting Komentar untuk "Clinical Trial Database Health Canada"