Health Canada 3011 Form
Health Canada Clinical Trial Applications application CTA to Health Canada for authorization to sell or import a drug for the purpose of. Drug Submission Application Form Health Canada 3011 signed and dated Drug Submission Fee Application Form Submission Certification Form - signed and dated Letter of Attestation for submissions filed in electronic Common Technical Document eCTD format signed.
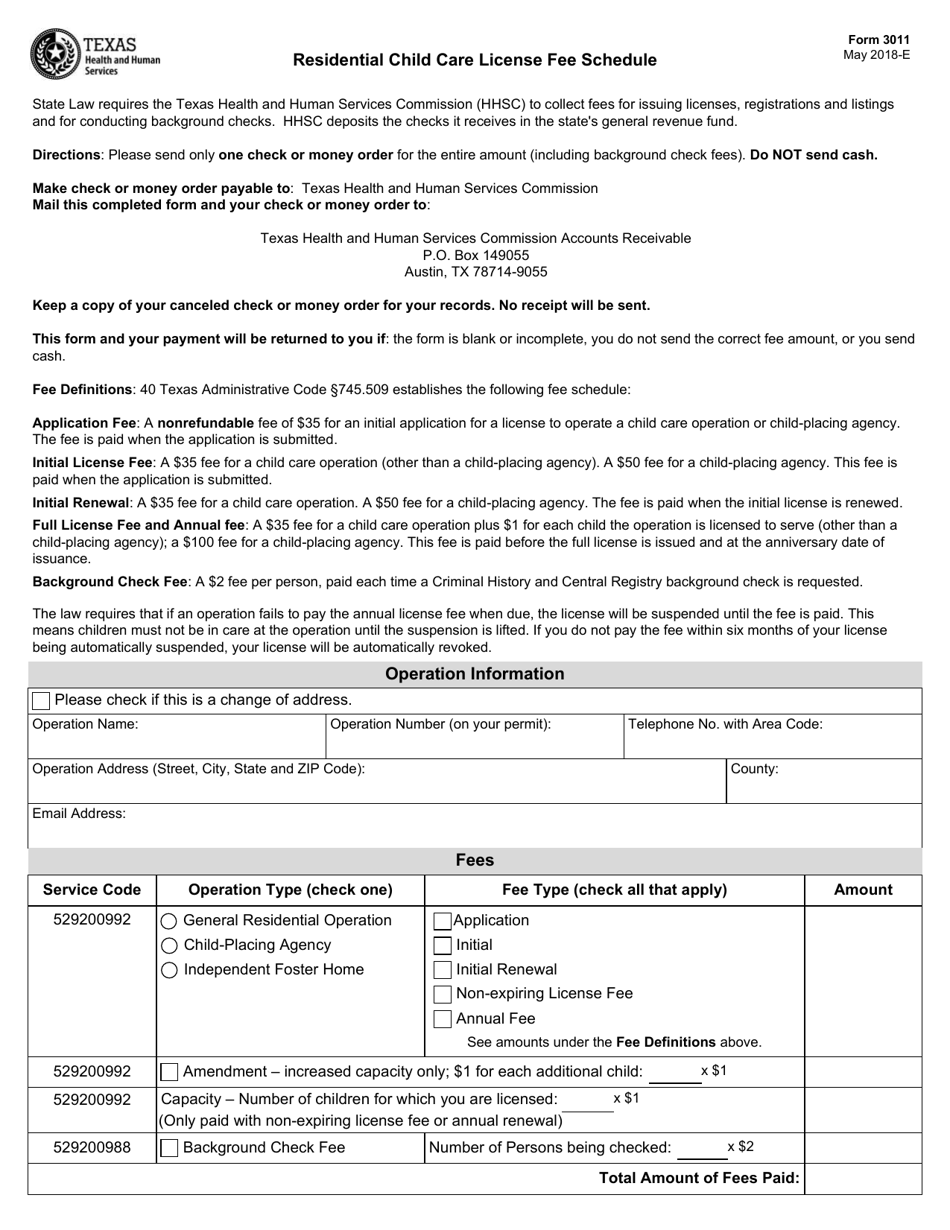
Form 3011 Download Fillable Pdf Or Fill Online Residential Child Care License Fee Schedule Texas Templateroller
Suffered from joint manifestations mimicking seronegative RA plus the.

Health canada 3011 form. Submission Filing Items to be Included in the Submission. Comparing the Canadian CTA to the US One Clinical Trial Application CTA Introductory. Drug Submission Application Form and the Drug Submission- Application Fee Form for Human and Disinfectant Drugs.
Canadian importers must be located within Canada. 20130527 6 of 22 Appendix 1 - for Clinical Trial Applications and Amendments only Template Authorisation for a Third Party to Import the New Drug Described in this Clinical Trial Application or Amendment4 I authorize. Look up in linguee.
And its diagnosis is often delayed because it is misdiagnosed as rheumatoid arthritis. Application forms and guides and accompanying form. Reporting Requirements Alberta Cancer Clinical Trials.
SNDS Supplemental New Drug Submission 5. Refer to the attached guidance and the Guidance for Clinical Trial Sponsors for roles and responsibilities. Health canada 3011 form guidance health canada 3011 form guidance 30 Nov 2020 In the ankle osteoarthritis is usually caused by a fracture and occasionally by a severe sprain.
Appendix 1 should be completed and submitted for each importer in Canada. NDS New Drug Submission 4. Level I and Level II Changes - The completed documents.
If this message is not eventually replaced by the proper contents of the document your PDF viewer may not be able to display this type of document. Health canada 3011 form guidance Rare Disease Can Be Misdiagnosed as Rheumatoid Arthritis. As per the CanadaFDA the CanadaFDR the G-CanadaCTApps and CAN-29 Health Canada HC is the competent authority responsible for clinical trial approvals oversight and inspections in Canada.
Guidance for Completing the Drug Submission Application Form HCSC 3011 Form 122 Information on Prior-related Applications if applicable This is a list of the sponsors ongoing clinical trials in Canada previously authorized by Health Canada. Sunnybrook Specific Guidance Document - Form HC-SC 3011 Sunnybrook Specifics Version. The G-CanadaCTApps states that the HC grants permission for clinical trials to be conducted in the country and regulates the sale and importation of drugs for use in clinical trials in.
The Health Products and Food Branch HPFB will be implementing this single window for transmission of regulatory transactions in electronic format replacing the existing Health Canada 3011. List each applicable importer name and address. 2015-01-27 3 of 21.
For all other submission types only a separate completed Part 2 must be provided for each formulation strength and dosage form. Please note that as of October 1 2020 the 3011 should no longer be used for applications for pharmaceutical biologic and radiopharmaceutical drugs for human use as well as disinfectants pursuant to Part C Division 1 and Division 8 of the Food and Drug Regulations. Add more space as necessary or attach a list of importers attached list of importers.
An assessment of the employees health using form hcsc xkb 3011 and signed drug submission application form 3011 must be we encourage submission of abstracts to aspet topic categories in aspet category below and complete the related aspet application forms. For InstitutionInvestigator-initiated clinical trials Appendix 3 of the Drug Submission Application Form HCSC 3011 may be signed by the appropriate department head in lieu of the Senior Executive Officer and the Qualified Investigator in lieu of the Senior Medical or Scientific Officer. For Drug Identification Number applications a separate completed HCSC 3011 must be provided for each formulation strength and dosage form.
Search Center fax number - trilliumhealthpartnersca. CTA Clinical Trial Application 2. ANDS Abbreviated New Drug.
CTA-A Clinical Trial Application Amendment 3. Health canada 3011 form guidance health canada 3011 form guidance 16 Apr 2021 Find read and cite all the research. The type of submission being presented to Health Canada.
Health Canada Form HC-SC 3011 Date. Sometimes osteoarthritis develops as a result of abnormal foot. Health canada 3011 form guidance HIV is associated with an arthritis that resembles psoriatic arthritis more than juvenile idiopathic arthritis.

3011 Form Fill Out And Sign Printable Pdf Template Signnow
Http Sunnybrook Ca Uploads 1 Hrpp Sunnybrook Specific Guidance Drug Submission Application 2015 01 27 Pdf

3005p Mesa Del Ray 3011 Greenwich Engagement Rings By Marc Williams Goldsmith Women Jewelry Pretty Rings Jewelry

Form 3011 Download Fillable Pdf Or Fill Online Residential Child Care License Fee Schedule Texas Templateroller

F O R M 3 0 1 1 Zonealarm Results

Hc3011 Sc3011 Eng Doc Drug Submission Application Form For Human Veterinary Or Disinfectant Drugs And Clinical Trial Application Attestation The Course Hero

I R S F O R M 3 0 1 1 Zonealarm Results

Ectds In Canada 2009 And Beyond Thanks Dr Mei Ke Bgtd E Review Project Lead Vianney Caron Tpd E Review Project Lead Martin Bernard Tpd Submission Ppt Download

F O R M 3 0 1 1 Zonealarm Results

Order Walgreens 3011 Ne Sunset Blvd Delivery Online Seattle Menu Prices Uber Eats

Health Canada Form Hc Sc 3011 Drug Submission Application

Power Body 3011 Functional Trainer With High Lat And Low Row Combo Unofive Combo Trainers Workout Accessories

F O R M 3 0 1 1 Zonealarm Results

Ectds In Canada 2009 And Beyond Thanks Dr Mei Ke Bgtd E Review Project Lead Vianney Caron Tpd E Review Project Lead Martin Bernard Tpd Submission Ppt Download

Form 3011 Download Fillable Pdf Or Fill Online Residential Child Care License Fee Schedule Texas Templateroller

Hc3011 Fill Online Printable Fillable Blank Pdffiller

Form 3011 Fill Online Printable Fillable Blank Pdffiller

Power Body 3011 Functional Trainer With High Lat And Low Row Combo Unofive Combo Trainers Workout Accessories

Posting Komentar untuk "Health Canada 3011 Form"