Health Canada Cta
Health Canada has stated that theres no regulatory requirement for the Office of Clinical Trials to issue Acknowledgement of Notification letters for Clinical Trial Application Notifications CTA-Ns. Clinical Trial Application CTA.
Comparing Ema And Health Canada Cta Submission For Phase I Trials To Us Fda Ind Application European Pharmaceutical Review
Health Canada recognizes that not all information required in the CTSI form may be available at the time of filing a CTA.
Health canada cta. The company has been keeping Health Canada informed through development of the vaccine candidate. Once the CTA review is complete Health Canada notifies the sponsor if the application is found to be acceptable or not. As set forth in the G-CanadaCTApps CAN-21 and CAN-23 HCs Health Products and Food Branch HPFB coordinates the CTA approval process.
Implementation of Clinical Trials Regulatory Activities in eCTD Format Begins by Health Canada After successful Clinical Trial regulatory activities in electronic Common Technical Document eCTD format in the pilot in August 2019 Health Canada begins implementation of Clinical Trials regulatory activities in eCTD format. Health Canada encourages submission of applications in Common Technical Document CTD format. Once a Clinical Trial Application CTA has been submitted questions can be raised by Health Canada to which an answer with or without commitment needs to be provided within 2 days.
Comparing the Canadian CTA to the US. Health Canadas Guidance Document. The cover letter should indicate.
Pre-Clinical Trial Application Consultation Meeting PRE-CTA Clinical Trial Applications CTAs with either a 7 day administrative or a 30 day default. For further information consult the Pre- CTA Consultation Meeting page. Health Canada invites sponsors to request a pre- CTA consultation meeting.
The EC review and approval process timeline vary by institution. Comparing the Canadian CTA to the US. Such consultations may be particularly useful for new active substances or applications that will include complex issues that may be new to Health Canada.
Once a CTA has been submitted and reviewed Health Canada notifies the Sponsor within 30 calendar days if the application is found to be acceptable or deficient. Once a CTA has been submitted and reviewed Health Canada notifies the Sponsor within 30 calendar days if the application is found to be acceptable or deficient. The G-CanadaCTApps and CAN-23 state that prior to initiating the trial the sponsor must file a CTA to the appropriate HPFB Directorate.
If the application is deemed acceptable a No Objection Letter NOL Guidance Document For Clinical Trial Sponsors. Clinical Trial Applications section 25 is issued by Health Canada. Despite this lack of any regulatory requirement the Office of Clinical Trials has been acknowledging about 4500 CTA-Ns each year.
CALGARY AB Dec. 4 2020 CNW - Providence Therapeutics confirmed today that a Clinical Trial Application CTA was submitted to Health Canada December 3 for the mRNA COVID vaccine PTX-COVID19-B. Health Canada is pleased to announce that the pilot was successful.
Health Canada is pleased to announce the release of the finalized Guidance Document for Clinical Trial Sponsors. Information from clinical trials is used in assuring the safety efficacy and quality of natural health products. If the application is deemed acceptable a No Objection Letter NOL is issued by Health Canada.
Note that the format of a CTA-A is similar to the format of a CTA. Clinical Trial Applications which provides guidance to all sponsors for example eg industry academic contract research organization seeking authorization to sell or import a drug for the purpose of a clinical trial in Canada. Sponsors are reminded that even if this information is not available when filing the CTA it is required prior to commencement of the trial as per C05006 1 d.
The NHPD is the directorate of Health Canada responsible for authorizing or denying permission to conduct a clinical trial for a natural health product. Health Canada has announced that as part of the Government of Canadas Regulatory Innovation Agenda for health products it will be modernising its clinical trial regulation framework to support the adoption of promising new therapies and match the accelerating advances in technology. Preparation of Drug Regulatory Activities in the Common Technical Document CTD Format and corresponding ICH guidance documents on the CTD format outline the modular structure and content of paper-based regulatory activities in CTD format.
Health Canada - General Investigational plan database of on going studies and sites - Investigator data statement CV- Protocol synopsis or rational product summary - 120 days to submit audited reports- Foreign Regulatory Authorities refusals -. Therefore implementation of Clinical Trials regulatory activities in eCTD format will begin immediately for the following. Please refer to section 273 for additional information.
This format as applied to a CTA-A Clinical is shown below.

Ct Angiography Cta Medicare Population Harvey L Neiman Health Policy Institute Study Neiman Hpi Interventional Cardiology Resume Health Policy

Best Practices Travel Industry Social Media Travel Industry Infographic Marketing Travel Health Insurance

15 Proven Call To Action Examples That Work In 2021 In 2021 Call To Action Examples Call To Action Action Words
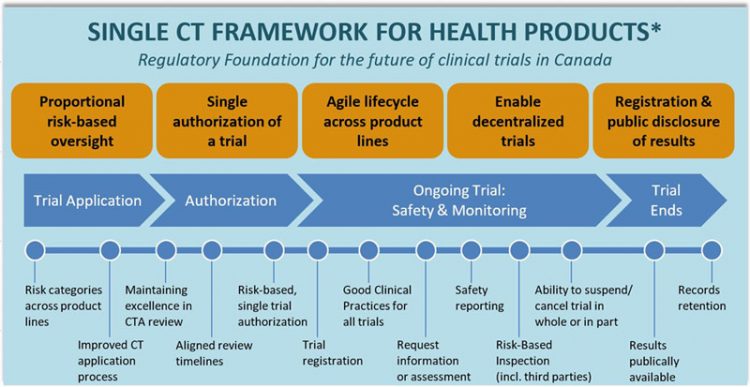
Health Canada To Modernise Its Clinical Trial Regulations

Pin On Blog Posts On A Chronic Voice

Guidance Document Creation Of The Canadian Module 1 Backbone

Call To Action Conference Is A 1 200 Attendee Digital Marketing Event In Vancouver Canada Created By Unboun Marketing Tactics Call To Action Digital Marketing

Cta Conf On Behance Event Marketing Marketing Tactics Unbounce

From Impd To Ind Same But Different Biopharma Excellence
Https Higherlogicdownload S3 Amazonaws Com Acrpnet E286ce54 127d 4eea 9c43 019995bcde22 Uploadedimages Ous 20 20canada Cpt Canada Webinar 20slides 092414 Pdf

Clinical Trial Approval Process In Canada Credevo Articles

Cta Wireframe Wireframe Wireframe Design Web Design

Health Canada Implements Ectd For Clinical Trials Regdesk

Comparison Of The Eu Cta And The Us Ind Application Procedures For Download Scientific Diagram

Canva Creating Calls To Action That Convert Social Media Call To Action Marketing Strategy Social Media

My Complete Blog Post Writing Process Checklist Paige Brunton Squarespace Templates Squarespace Designer Courses In 2021 Blog Post Checklist Blog Post Template Writing Blog Posts

Comparing Ema And Health Canada Cta Submission For Phase I Trials To Us Fda Ind Application European Pharmaceutical Review


Posting Komentar untuk "Health Canada Cta"