Health Canada Medical Device Database
Establishment Licensing EL fees Health Canada inspects establishments to assess whether they comply with regulatory requirements to conduct regulated activities related to drugs and medical devices. Although some information on drugs and medical devices can be accessed via alternate channels Health Canada encourages stakeholders to use the databases as a primary means of accessing information.
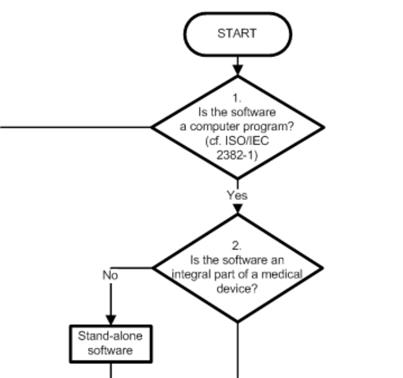
Software As Medical Device Samd Classification And Definitions
The same device may have different names in different countries.
Health canada medical device database. The Bureau maintains a database of all licensed Class II III and IV medical devices offered for sale in Canada. Report a serious adverse drug reaction for hospitals Report a medical device problem for health care professionals Prescription Drug List. Generic drug manufacturers must update their PM to ensure it aligns with the Canadian Reference Product.
This action is similar to the FDAs proposed rule for the regulation of Medical Device Data Systems MDDS nearing finalizationThe Canadian announcement begins with a reminder of its definition of medical device which is similar to. Only products which appear in this database listing may be offered for general marketing purposes in Canada. The MDL is a product approval while a MDEL is a permit for the companydistributorimporter itself.
Health Canadas COVID -19 Medical Device Database Can Be Very Misleading November 26 2020 By dicentra If you are involved in the importation or distribution of Medical Devices related to the COVID crises in Canada then no doubt you are aware of Health Canadas online database that goes under the name Authorized medical devices for uses related to COVID-19. Before a drug or medical device is authorized for sale in Canada Health Canada reviews it to assess its safety efficacy and quality. The Bureau maintains a database of all licensed Class II III and IV medical devices offered for sale in Canada.
Obtaining an MDL is comparable to the US FDA 510 k process. Product monograph PM for human drugs. Adverse reaction reports are submitted by.
This window is identical to the original MDALL search and. Report a side effect. An MDEL provides Health Canada assurance that medical devices sold or imported into Canada meet the safety requirements set out in the Medical Devices Regulations and that procedures are in place to protect the public should a problem with a device.
We are not suggesting or implying that any companies or other entities included in the International Medical Devices Database engaged in unlawful conduct or otherwise acted improperly. Medical devices help to diagnose prevent and treat many injuries and diseases. Class I medical devices do not require a medical device licence and are monitored by the Health Products and Food Branch Inspectorate Compliance and Enforcement through Establishment Licensing.
Medical Devices Active Licence Listing MDALL - Your reference tool for licensed medical devices in Canada. Health Canada Medical Device License MDL A Canadian Medical Device License MDL is required for companies selling Class II - IV medical devices in Canada. Search changes in manufacturer ownership so it reflects HC database Check MDALL Medical Device Active Licence Listing for device class and.
Data and review decisions. Health Canada Medical Device. Submission of inadequate incident reports by reporters for which Canada Vigilance - Medical Device Problem Reporting Program is consistently required to request additional information will result in the forwarding of this information to the Health Products and Food Branch Inspectorate to determine regulatory compliance.
Availability of the drug in Canada. Canada Vigilance adverse reaction online database For industry information about COVID-19 visit our COVID-19 health product industry section. A Medical Device Establishment Licence MDEL is a licence issued to Class I manufacturers as well as importers or distributors of all device classes to permit them to import or distribute a medical device in Canada.
About the Drug and Health Product Register. Class I medical devices do not require a medical device licence and are monitored by the Regulatory Operations and Regions Branch Compliance. Dear visitor We have reorganized our Web site.
Labels for animal drugs. Health Canadas drug and medical device databases are intended to increase transparency and to make information accessible to stakeholders and the general public. Sells a medical device in Canada for the purpose of resale or use other than for personal use.
Search the Drug Product Database DPD to find drugs authorized for sale by Health Canada. A person outside of Canada selling. The DPD is updated nightly and includes.
Selecting the Active Licence Search link takes you to the Medical Devices Active Licence Search window. The Canada Vigilance Adverse Reaction Online Database contains information about suspected adverse reactions also known as side effects to health products. On August 31 2009 Health Canada Canadas medical device regulatory authority posted classification information for Patient Management Software pdf.

Covid 19 Diagnostic Tests Production Gaps Bioprocess Internationalbioprocess International
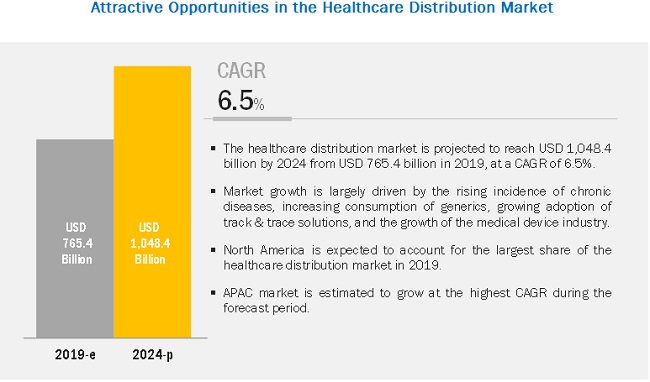
Healthcare Distribution Market Global Forecast 2024 Marketsandmarkets

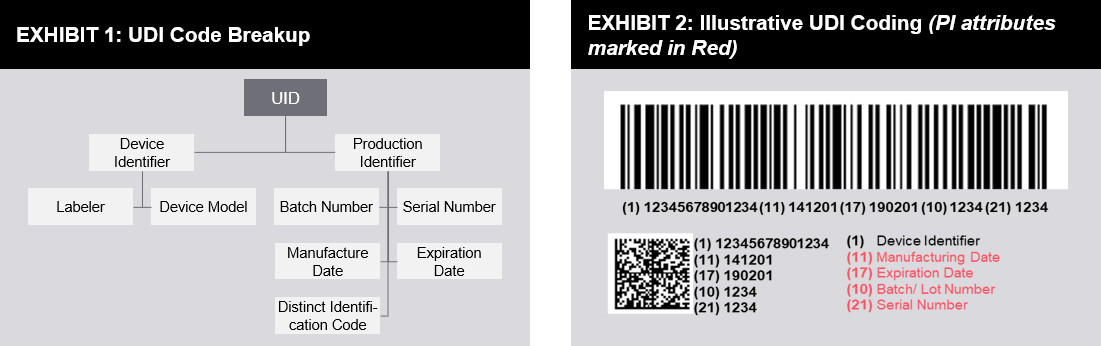
Unique Device Identification Udi For Medical Devices Futurebridge
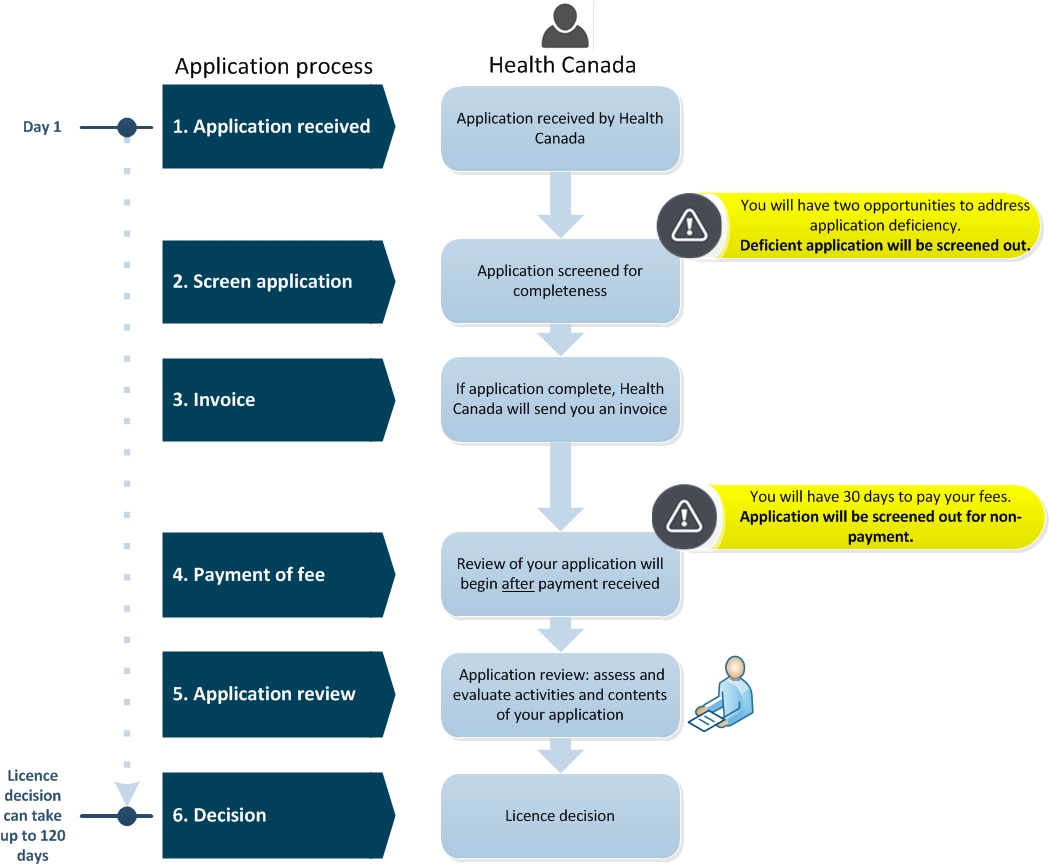
Frequently Asked Questions Medical Device Establishment Licensing And Fees Canada Ca
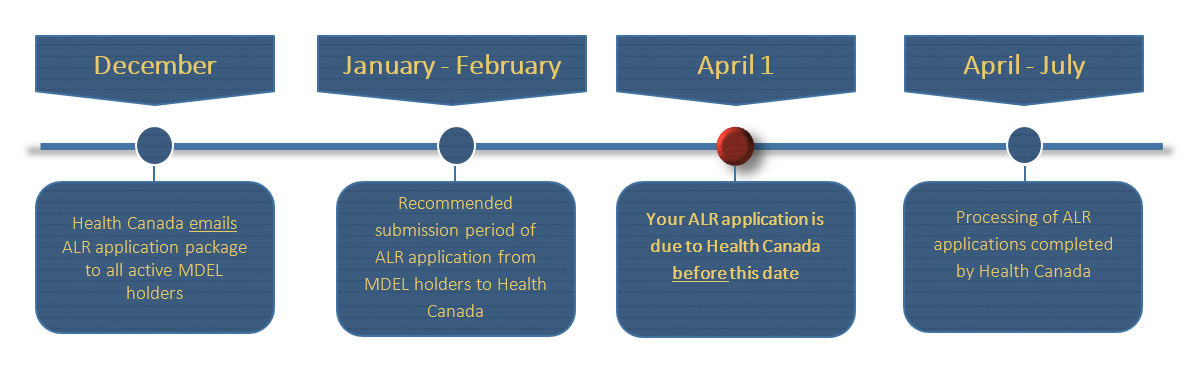
Frequently Asked Questions Medical Device Establishment Licensing And Fees Canada Ca

Medical Devices Have Caused More Than 80 000 Deaths Since 2008 Stat

Software As Medical Device Samd Classification And Definitions

Clinical Trials Medical Device Trials Genesis Research Services
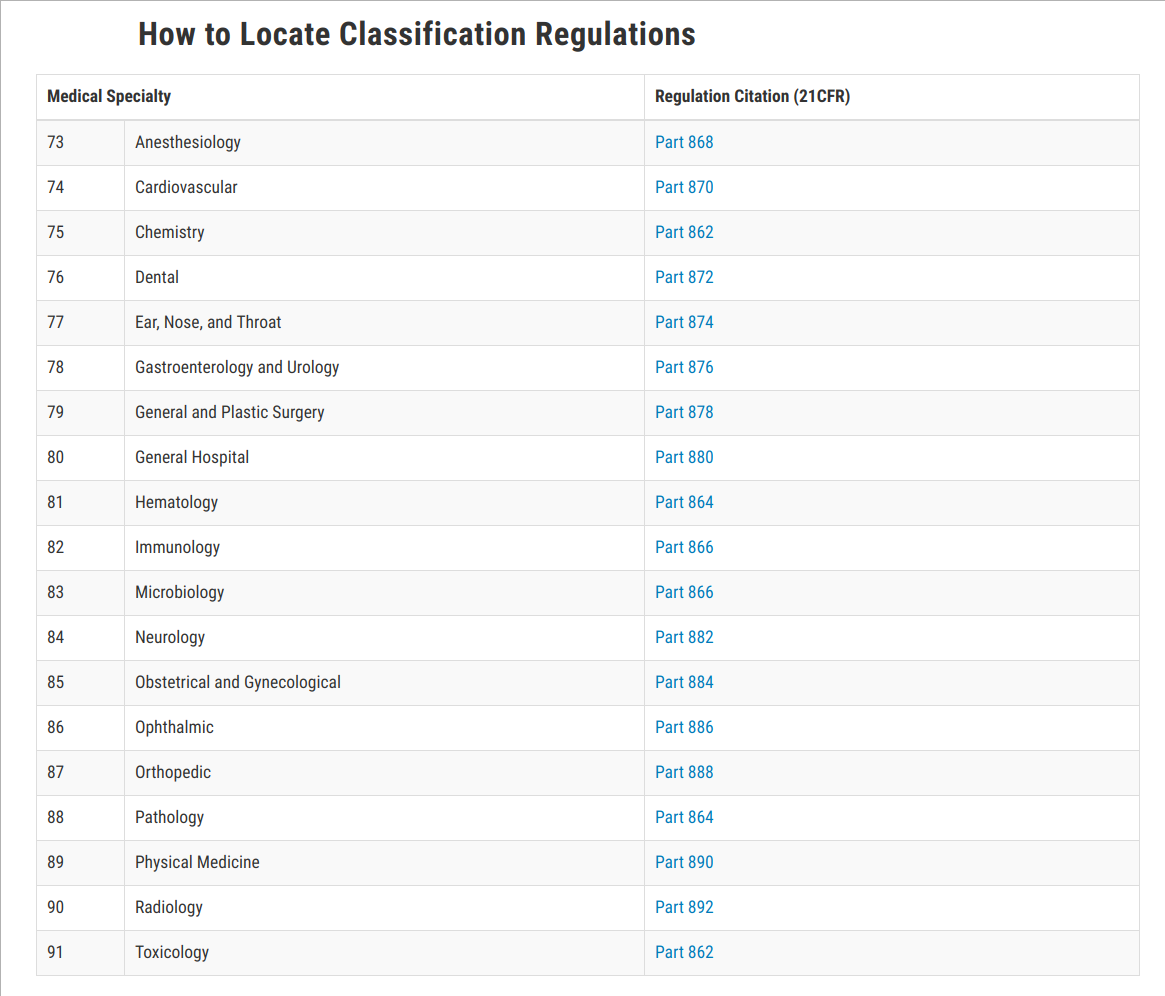
Does An Fda Class 1 Medical Device List Exist
Software As Medical Device Samd Classification And Definitions

Incident Reporting For Medical Devices Guidance Document Canada Ca

Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca

Unique Device Identification Udi For Medical Devices Futurebridge
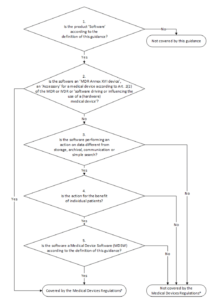
Software As Medical Device Samd Classification And Definitions
Drug Database Medical Device Database Fdb First Databank
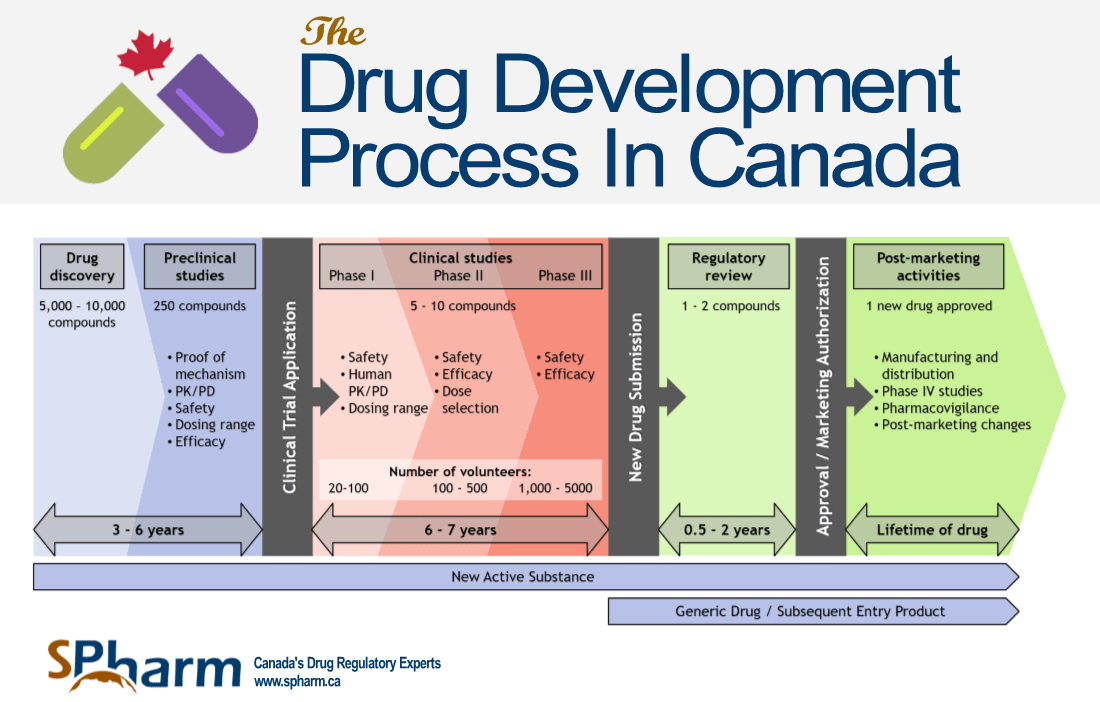
The Drug Review And Approval Process In Canada An Eguide Spharm Canada S Drug Regulatory Experts

Complete Guide To Bringing A Medical Device To Market
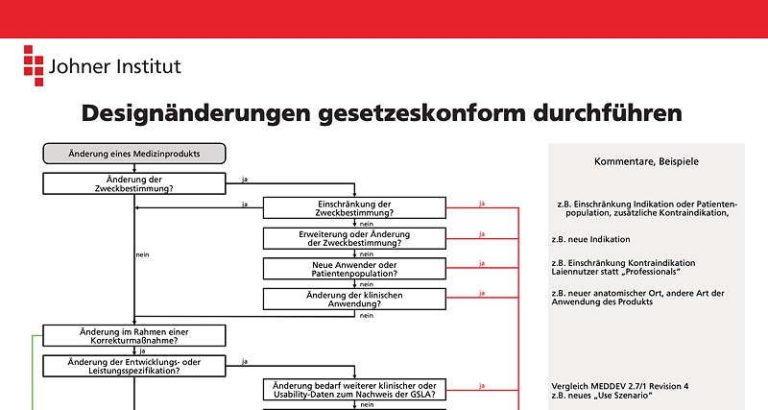
Design Change What It Is And When Re Approval Is Required
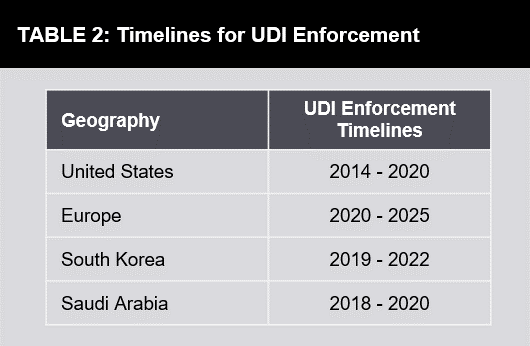
Unique Device Identification Udi For Medical Devices Futurebridge

Posting Komentar untuk "Health Canada Medical Device Database"