Health Canada Regulatory Review Of Drugs And Devices
We understand the particular needs and challenges of Canadians with rare diseases and have made a commitment to improve access to medications that treat these conditions. In December 2018 HEALTH CANADA released the Action Plan on Medical Devices entitled Continuously Improving Safety Effectiveness and Quality.

Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca
This project aims to improve our ability to assess and monitor the safety efficacy and effectiveness of drugs across the drug life cycle.

Health canada regulatory review of drugs and devices. Latest developments on drugs and health products related to COVID-19. It will also help us to develop new regulatory options to prevent. Improving the regulatory review of drugs and devices stakeholder meetings and engagement activities In keeping with our commitment to openness and transparency a notice was posted to inform stakeholders on how Health Canada will handle meeting and correspondence information for specific initiatives including Improving the regulatory review of drugs and devices.
Aligned reviews for certain drugs. Strengthening the use of real world evidence for drugs. SBDs are written in technical language for stakeholders interested in product-specific Health Canada decisions and are a.
This project will result in. The Health Canada and Health Technology Assessment HTA review processes are independent of each other. For a drug a biologic or a genetic therapy a medical device a combination product a natural health product or other health product company seeking approval of their product for sale in Canada it is important to understand that the approval process is subject to close scrutiny by the governing regulatory body.
Health Canadas Summary Basis of Decision SBD documents outline the scientific and regulatory considerations that factor into Health Canada regulatory decisions related to drugs and medical devices. Improving the regulatory review of drugs and devices. Health Canada has also introduced regulatory measures to address drug shortages.
Health Canadas Regulatory Review of Drugs and Devices initiative aims to provide more timely access to drugs and devices. Under this initiative greater collaboration is being sought between organizations that play a role in drug access including Health Canada and the health technology assessment HTA organizations the Canadian Agency for Drugs and Technologies in. Improving the regulatory review of drugs and devices.
Improving the regulatory review of drugs and devices. Early scientific advice to manufacturers. The Regulatory Review of Drugs and Devices Initiative will make regulatory processes more efficient and better able to meet the needs of the health care system.
Updated requirements for COVID-19 drug authorizations. This project will make sure that the timing of Health Canada and Canadian HTA reviews are better aligned while facilitating information sharing between Health Canada and HTA organizations. Engagement with counterpart regulatory agencies and Health Technology Assessment HTA.
We are therefore giving the option to sponsors to formally align reviews of all qualifying submissions in partnership with. This project aims to improve our ability to monitor medical device safety and effectiveness across the product life cycle. Health Canadas regulatory response to COVID-19.
Once a drug is developed Health Canada must give an authorization before the drug can be marketed. Strengthening the use of real world evidence and regulations for medical devices. A targeted Health Canada review for digital health technologies.
This includes meeting the needs of people with rare diseases. Health Canadas Regulatory Review of Drugs and Devices R2D2 initiative aims to provide more timely access to medicines for Canadians. We are committed to openness and transparency and we want you to have your say throughout the process.
This project was launched as part of Health Canadas Regulatory Review of Drugs and Devices R2D2 initiative. Published on July 16 2021 On May 20 2021 Health Canada opened a consultation on its proposal to modernize the regulatory framework for clinical trials related to human drugs medical devices non-prescription drugs and natural health products to seek feedback from key stakeholders to validate and inform further policy development. Interim order respecting the importation and sale of medical devices for use in relation to COVID-19 2020-03-18.
Building better access to digital health technologies. We are improving the regulatory review process of drugs and medical devices in Canada. Greater capacity for digital health technologies especially emerging innovations such as artificial intelligence and telerobotics.
The outcome for the people of Canada will be faster access to drugs including drugs for rare diseases. This project will build new review pathways for those drugs shown to meet health care system needs. Stakeholder engagement sessions now to March 2018 with.
Health Canadas transparency. We will host consultation sessions both in-person and online ending in 2021. One of these measures was an interim order to prevent or ease shortages of drugs medical devices and foods for a special dietary purpose It was originally introduced on March 30 2020.
Regulatory transparency and openness. After market authorization the HTA organizations give drug funding recommendations to. The tasks for this project include.
Access to health products 2020-07-03. The plan briefly outlines a three-part strategy to continue to improve the safety.
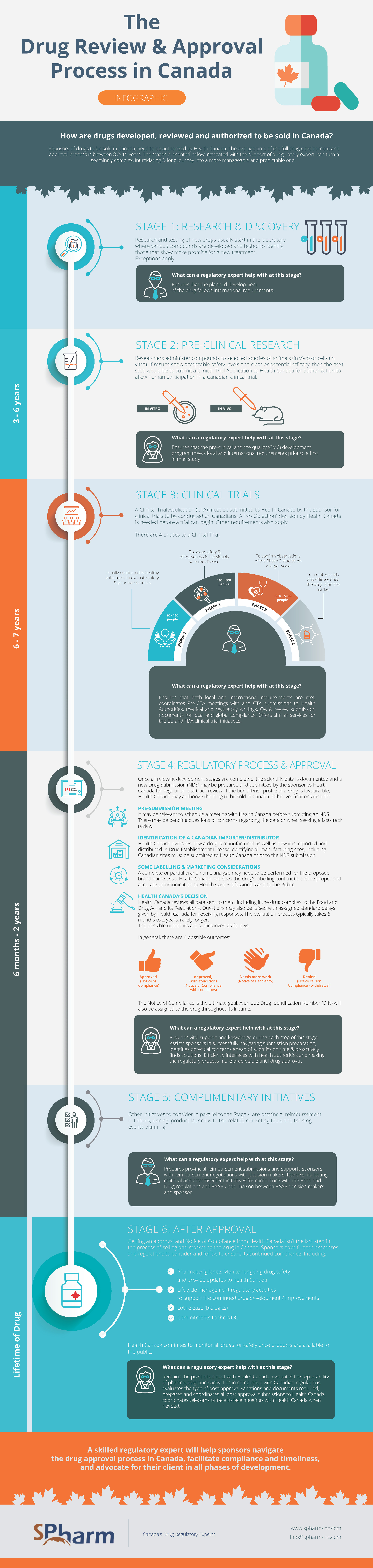
The Drug Review And Approval Process In Canada An Eguide Spharm Canada S Drug Regulatory Experts

Guidance For Industry Keyword Index To Assist Manufacturers In Verifying The Class Of Medical Devices Medical Device Medical Manufacturing

Regulatory Medical Writing Infographic Medical Regulatory Writing

Drug And Medical Device Highlights 2018 Helping You Maintain And Improve Your Health Canada Ca
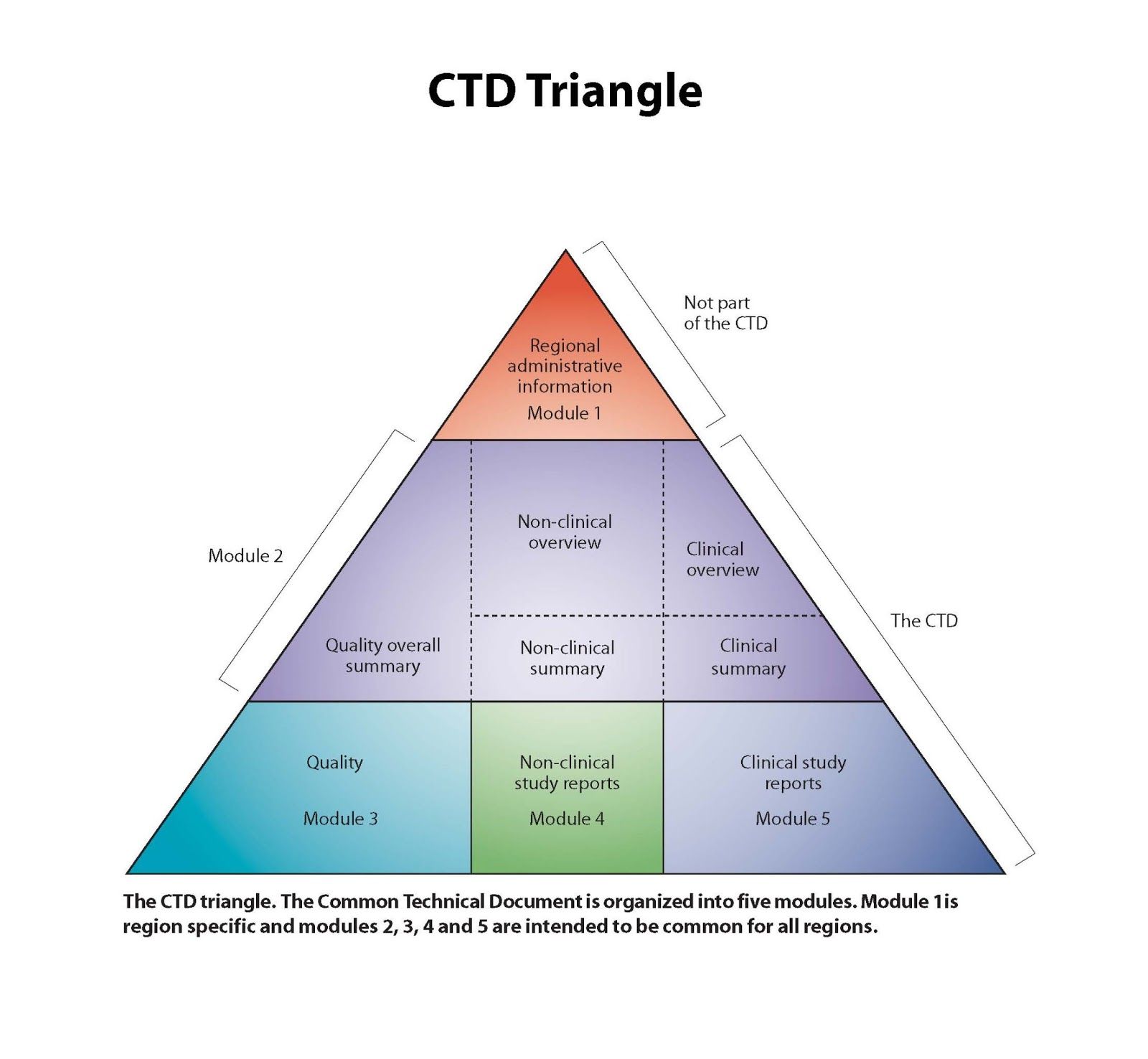
The Drug Review And Approval Process In Canada An Eguide Spharm Canada S Drug Regulatory Experts

The Drug Review And Approval Process In Canada An Eguide Spharm Canada S Drug Regulatory Experts

Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca

The Drug Review And Approval Process In Canada An Eguide Spharm Canada S Drug Regulatory Experts

Pin On Professional Quality And Regulatory

Pin By Vuvu On Programmes Office Health Care Pharma Pharmaceutical Company

What Is A Medical Device Official Definition For Eu Usa China Brazil Medical Medical Device Medical Equipment

Clinical Trials Medical Device Trials Genesis Research Services

Glymph Healthcare Medical Tourism Home Health Agency Healthcare Marketing
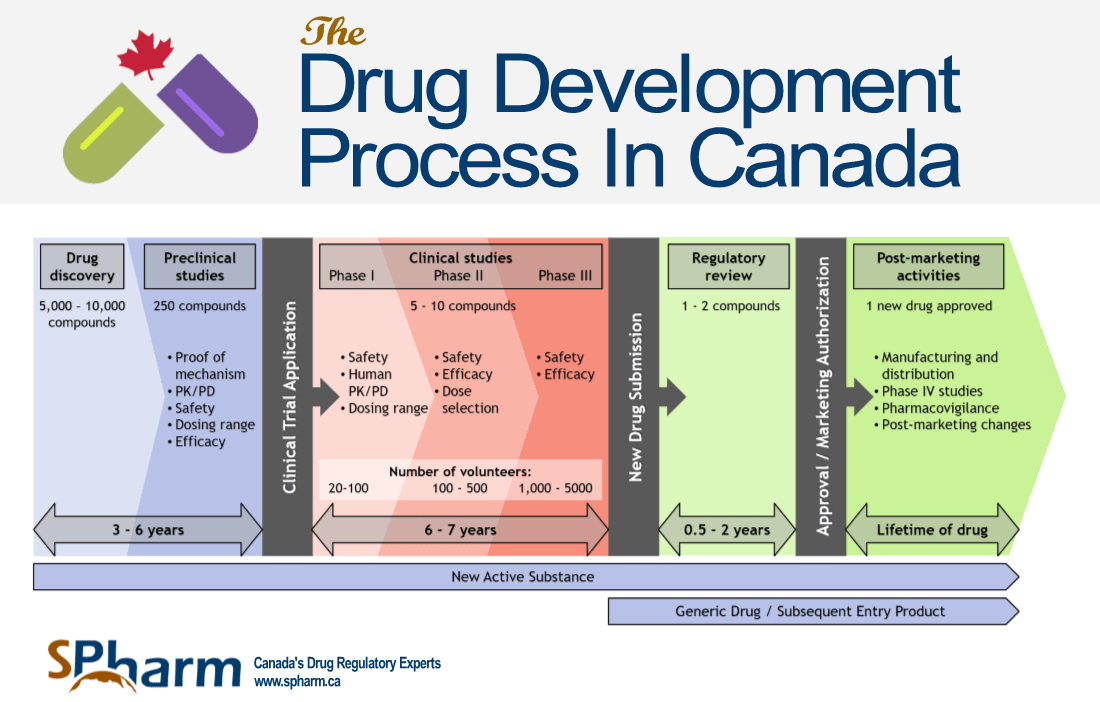
The Drug Review And Approval Process In Canada An Eguide Spharm Canada S Drug Regulatory Experts

The Drug Review And Approval Process In Canada An Eguide Spharm Canada S Drug Regulatory Experts




Posting Komentar untuk "Health Canada Regulatory Review Of Drugs And Devices"