Clinical Trial Application Guidance Health Canada
Guidance document 2020-05-27 Interim Order Respecting Clinical Trials for Medical Devices and Drugs Relating to COVID-19. Clinical Trial Applications CTA s.
Identification Of Regulatory Needs For Nanomedicines Bremer Hoffmann 2018 Journal Of Interdisciplinary Nanomedicine Wiley Online Library
Health Canada is pleased to announce the release of the guidance document Quality Chemistry and Manufacturing Guidance.
Clinical trial application guidance health canada. Applications for drug clinical trials under the Interim Order. Access Workspace for Care. Guidance Guidance Document for Clinical Trial Sponsors.
CTA filing requirements for these types of trials should include the information as described in Section 52 with the following modifications. March 12 2021 0 Comments. If the manufacturer of that product has previously submitted information to Health Canada that meets the regulatory requirements for CTAs a letter authorizing cross reference to their information on file may be submitted in lieu of.
Clinical Trial Applications - Open Government Portal Open Data will consolidate 59515 Canada Lands Survey records from Natural Resources Canadas Survey Plan Search Service into one dataset. Health Canada guidance documents to assist in the interpretation of policies and governing statutes and regulations when preparing drug submissions when seeking an approval to sell a drug product in Canada. Currently Health Canada may not always be aware of third parties involved in a trial given that there is no requirement for sponsors to provide information on the involvement of CROs and SMOs to Health Canada although information on QIs is supplied as part of the clinical trials site information CTSI form or as part of the Investigational Testing Application for medical devices.
Clinical Trial Applications G-CanadaCTApps Last Revised March 17 2016 Health Products and Food Branch Health Canada Guidance Guidance Document. Guidance documents - Biologics Radiopharmaceuticals and Genetic Therapies. Info You Need When You Need It.
Guidance Document For Clinical Trial Sponsors. Please do not provide forms until all fields are completed. Additional details on requesting a meeting and meeting.
Ad Our Software Can Be Configured To Suit Your Organisations Individual Needs. Dates for sections 35 and 47 of the CTSI Form must be provided. Ad Our Software Can Be Configured To Suit Your Organisations Individual Needs.
Health Canada is pleased to announce the release of the finalized Guidance Document for Clinical Trial Sponsors. Clinical Trial Applications CTA s for Pharmaceuticals and three Quality Overall Summary Chemical Entities Clinical Trial Applications templates. A completed Clinical Trial Site Information Form CTSI Form for each proposed clinical trial site if known at the time of the application is to be submitted.
Use of pharmacometrics in drug submissions and clinical trial applications 2021-03-31. Format of clinical trial application The sponsor must file a CTAP in the format required by Health Canada. As per the CanadaFDA the CanadaFDR the G-CanadaCTApps and CAN-29 Health Canada HC is the competent authority responsible for clinical trial approvals oversight and inspections in Canada.
In addition the sponsor may also utilize their cover page to provide additional and relevant information. Certain clinical trials involving drugs medical devices or natural health products may require an application for regulatory approval be filed with Health Canada prior to. Notice 2020-05-27 Clinical trials guidance.
As a rule the appl ication must be organized into three different modules each containing the following information. The G-CanadaCTApps states that the HC grants permission for clinical trials to be conducted in the country and regulates the sale and importation of drugs for use in clinical trials. Per the G-MDSA and the G-CanadaCTApps sponsors may request a pre-submissionapplication meeting with the appropriate Directorate within HPFB if they have any questions or concerns prior to filing a CTA.
The following guidance documents have been prepared to assist in the interpretation of the policies governing statutes and regulations. Management of Drug Submissions and Applications G-MDSA Effective April 1 2020. 22 Module 1 contains administrative and clinical information about the proposed trial.
Clinical Trial Applications which provides guidance to all sponsors for example eg industry academic contract research organization seeking authorization to sell or import a drug for the purpose of a clinical trial in Canada. The sponsor must file a clinical trial application CTA to the appropriate Directorate within HCs Health Products and Food Branch HPFB. Clinical Trial Applications for Pharmaceuticals.
Info You Need When You Need It. The Guidance was revised based on stakeholder consultation processes and as part of Health Canadas. Access Workspace for Care.
This guidance document supersedes the previous Health Canada draft guidance document Revised Draft Quality Chemistry and Manufacturing Guidance. Overview of the Mandatory reporting of serious adverse drug reactions and medical device incidents by hospitals.
Https Ethics Research Ubc Ca Sites Ore Ubc Ca Files Documents Policy 20on 20form 201572 20may 2015 202017 Pdf
Https Ethics Research Ubc Ca Sites Ore Ubc Ca Files Documents E 20consent 20final 20april 2029 20with 20links Pdf
Application Requirements For Cannabis Cultivation Processing And Medical Sales Licences Canada Ca
Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca
Guidance Document Part C Division 5 Of The Food And Drug Regulations Drugs For Clinical Trials Involving Human Subjects Gui 0100 Canada Ca
Post Authorisation Safety Studies Pass European Medicines Agency
Drug And Natural Health Products Recall Guide Canada Ca
Application Requirements For Cannabis Cultivation Processing And Medical Sales Licences Canada Ca
Nabilone For Chronic Pain Management A Review Of Clinical Effectiveness And Guidelines An Update Ncbi Bookshelf
Covid 19 Diagnostic Tests Production Gaps Bioprocess Internationalbioprocess International
Comparison Of Regulatory Frameworks Of Environmental Risk Assessments For Human Pharmaceuticals In Eu Usa And Canada Sciencedirect
Post Authorisation Safety Studies Pass European Medicines Agency
Health Canada Guidance On Io Applications Regdesk
Covid 19 Diagnostic Tests Production Gaps Bioprocess Internationalbioprocess International
Health Canada Guidance On Io Applications Regdesk
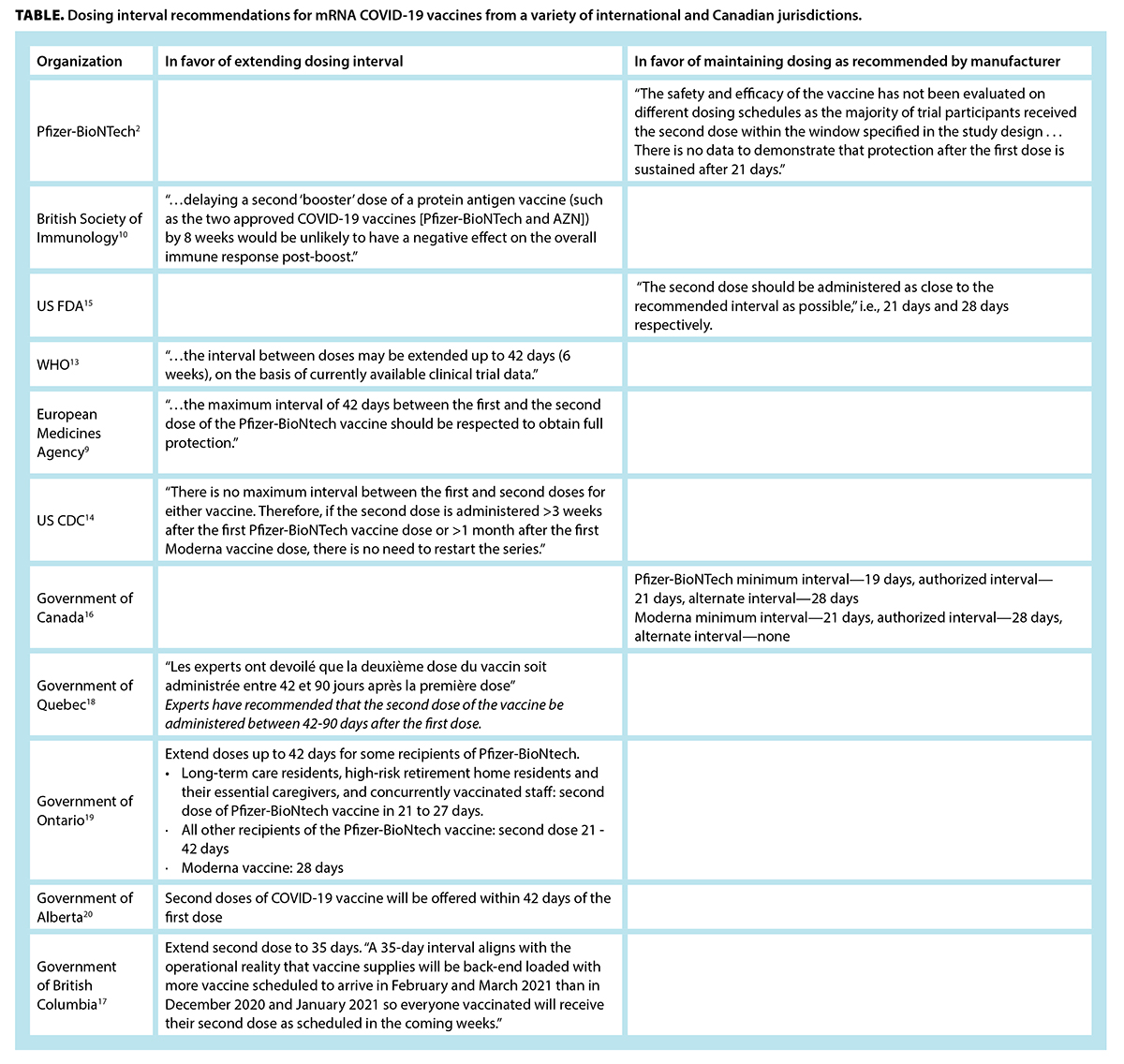
What Is The Evidence For Extending The Sars Cov 2 Covid 19 Vaccine Dosing Schedule British Columbia Medical Journal
Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca



Posting Komentar untuk "Clinical Trial Application Guidance Health Canada"