Health Canada Device Listing
Fees for the Right-To-Sell RTS licensed class II III or IV devices Right-To-Sell medical device The annual fee to right to sell medical devices class II III or IV. Cannot advertise or represent by label a treatment for a Schedule A disease or disorder Section 3 Cannot sell or advertise a device that may cause harm Cannot sell or advertise a device in a misleading or deceptive way All medical devices those used on human beings must also comply.
The Medical Devices Bureau Bureau of the Therapeutic Products Directorate Health Canada is the Canadian federal regulator responsible for licensing medical devices in accordance with the Food and Drugs Act and Regulations and the.
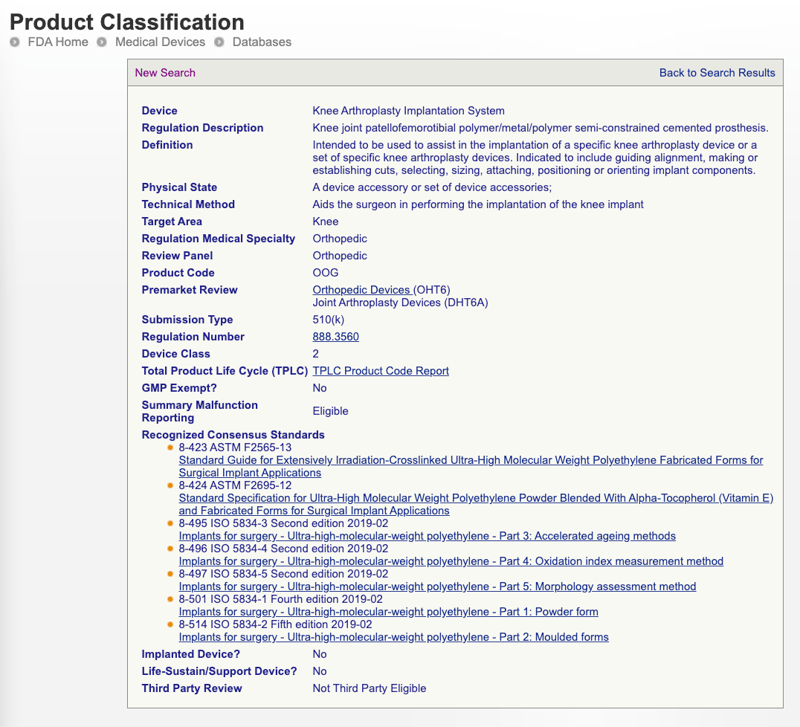
Health canada device listing. 10222020 Active licence listing by company httpshealth-productscanadacamdall-limhdispatch-repartitiondotypeactive 57 Device first issue date Device name Identifier first issue date Device identifier 2015-08-05 CEG1004 2015-08-05 CEG1005 2015-08-05 CEG1006 Licence No. Please select all that apply. There is an annual fee for the Right to Sell a Class II III IV Medical Device.
An MDEL provides Health Canada assurance that medical devices sold or imported into Canada meet the safety requirements set out in the Medical Devices Regulations and that procedures are in place to protect the public should a problem with a device be identified. Health Canada originally mandated that these companies would be required to register their companies to the ISO 13485 standard as the software is a medical device. 381 20 days to update Medical Device Licence Listing database following receipt of a complete Annual Notification Package Other finalized significant changes in fees.
Health Canada will continue to update this notice as more information becomes available. In Canada medical devices are classified into one of 4 classes. There are four device classifications--Class I II III and IV--using a set of 16 rules found in Schedule 1 Part 1 of the Canadian Medical Devices Regulations CMDR SOR98-282.
Medical Devices Active Licence Listing MDALL - Your reference tool for licensed medical devices in Canada. Medical Devices Active Licence Listing MDALL Purchase of Licensed Medical Devices for Use in Health Care. The process of securing an MDL is usually faster than that a 510 k.
Health Canada monitors medical devices on the Canadian market through post-market surveillance and compliance and enforcement activities. The Bureau maintains a database of all licensed Class II III and IV medical devices offered for sale in Canada. Report a problem or mistake on this page.
To determine the appropriate classification for their device manufacturers are encouraged to refer to the classification rules for medical devices in the Medical Devices Regulations. Medical Devices Active Licence Listing MDALL Health 5 days ago Medical Devices Active Licence Listing MDALL - Your reference tool for licensed medical devices in CanadaFrom Health CanadaDear visitor We have reorganized our Web site. Its now looking like.
Class I medical devices do not require a medical device licence and are monitored by the Health Products and Food Branch Inspectorate Compliance and Enforcement through Establishment Licensing. The MDEL listing contains information about the licensed establishment including their company ID licence number company. Fees for the right to sell medical devices.
Check whether medical devices have been authorized for sale by searching Health Canadas Medical Devices Active Licence ListingMDALL. Selecting the Active Licence Search link takes you to the Medical Devices Active Licence Search window. Obtaining an MDL is comparable to the US FDA 510 k process.
Regulatory Provisions All devices offered for sale in Canada must comply with the Food and Drugs Act. For all other medical devices guidance documents and forms for applications please. Medical Devices Licence Listings.
Dear visitor We have reorganized our Web site. A Canadian Medical Device License MDL is required for companies selling Class II - IV medical devices in Canada. Selecting the Active Licence Search link takes you to the Medical Devices Active Licence Search window.
IVDs are also classified as Class I through IV using a set of 9 rules which can be found in Schedule 1 Part 2 of the CMDR. Class I represents the lowest risk and Class IV the highest. A link button or video is.
Medical Devices Active Licence Listing MDALL - Canada. Health Canada is the federal regulator of therapeutic products including medical devices in Canada. Also check Recalls and Safety Alerts database for advisories on illegal health products that have been found on the Canadian market.
Device Family Device class. Only products which appear in this database listing may be offered for general marketing. Hello all Just an FYI for all those companies manufacturing electronic medical records software.
To obtain further information regarding medical device licensing or to submit an application for authorization under the Interim Order for COVID-19 medical devices please contact the Medical Devices. The MDL is a product approval while a MDEL is a permit for the companydistributorimporter itself.
Enter A Certificate To Foreign Government Cfg Application Step By Step Instructions
Guidance On Medical Device Establishment Licensing Gui 0016 Canada Ca
Revised Fee Proposal For Drugs And Medical Devices Canada Ca
Frequently Asked Questions Medical Device Establishment Licensing And Fees Canada Ca
Benefits Of Using Iec 60601 Compliant Components In Medical Devices
Complete Guide To Bringing A Medical Device To Market
Transfer Ownership Of Devices And Facilities
The Purpose Of This Procedure Is To Define Company Name S
Guidance Document Software As A Medical Device Samd Classification Examples Canada Ca
Private Labeled Devices With Fda Approval Medical Device Academy Medical Device Academy
Frequently Asked Questions Medical Device Establishment Licensing And Fees Canada Ca
Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca
Private Labeled Devices With Fda Approval Medical Device Academy Medical Device Academy
Https Www Who Int Medical Devices Countries Regulations Can Pdf Ua 1
Does An Fda Class 1 Medical Device List Exist
The Definitive Guide To Ifu For Medical Devices Eu Us
Private Labeled Devices With Fda Approval Medical Device Academy Medical Device Academy


Posting Komentar untuk "Health Canada Device Listing"