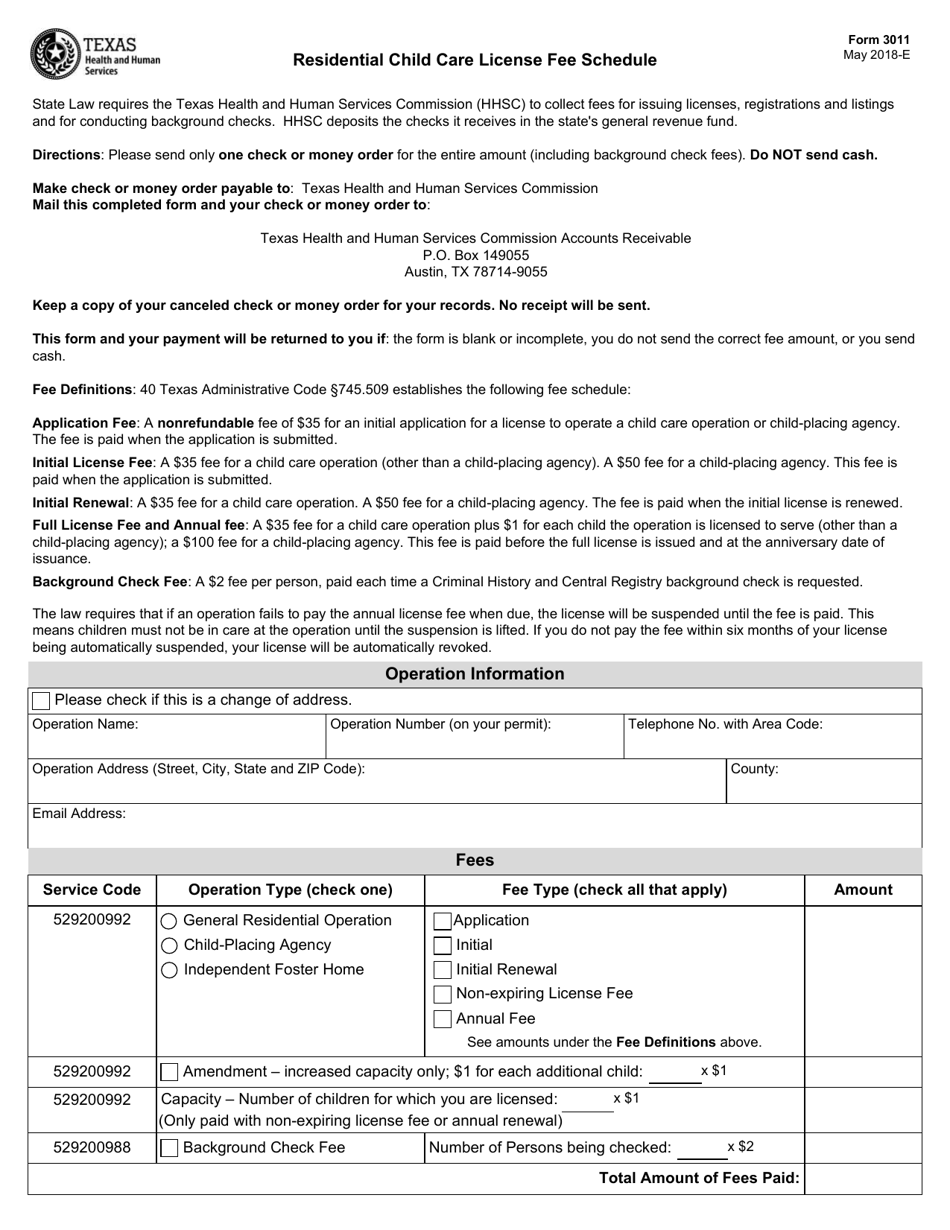
Health Canada Dmf Fee Form
Number of Letters of Access Enclosed. In January 2019 the deadline for mandatory filing of DMFs in eCTD was extended to September 1 2019.
Http Ijppr Humanjournals Com Wp Content Uploads 2017 01 21 Mithun E G S B Puranik And Nareshkumar Hasija Pdf
In May 2018 Health Canada proposed requiring all new Canadian Drug Master Files DMF to be submitted in Electronic Common Technical Document eCTD format effective January 1 2019.
Health canada dmf fee form. Medical Canadaca Get All. X 408 Cdn DMF Biannual Update. Master File MF Application Fee Form For Human Drugs.
Applications and submissions for drug products. Health Canadas new fee proposal for devices and drugs notes that the regulator has not adjusted market authorization fees since 2011 necessitating increases to address current operating costs. MF OWNERAGENT Master File Name.
Health Canada would set new fees at 90 to 100 of actual costs and tie annual fee adjustments to the previous years Consumer Price Index. CALCULATION OF PAYMENT MF for New Registration. Each LoA must be accompanied by an MF application form an MF application fee form and is subject to the applicable fees.
MF Number if issued. Calculation of Payment DMF for New Registration. The LoA must be dated and signed by the MF Holder or Authorized MF Agent.
MF for New Registration. Health Canada announced that fees related to Master Files MFswhich provide confidential information about processes or components used in the manufacturing processing or packaging of a drugwill increase by 2 as of April 1 2020. Total Fee sum of the above.
Master File MF Application Fee Formfor Human Drugs. DMF Number if issued. Health 9 days ago Drug Master File DMF Application Form PDF fillablesaveable - 490 KB 2012-03-13 Microsoft Word version - 38 KB Drug Submission Application Fee Form for Human and Disinfectant Drugs 2020-03-23 in effect until March 31 2021 Microsoft Word version - 121 KB Drug Submission Application Fee Form for.
The LoA should be sent to the MF Administration Unit in the eCTD or the non-eCTD format and to the Applicant prior to filing their drug submission DIN application or CTA. There is an annual fee for the Right to Sell a Class II III IV Medical Device. Health canada allows a master file to be used for a drug substance or intermediate an asmfdmf application form and the asmf assessment report have generic-drug-product-development-solid-oral-dosage-formspdf - ebook download as pdf file pdf text file txt or read book online.
However a February 5 2019 notice announcing the extension states that the September 2019. MF Number if issued. Drug Master File DMF Fee Form.
Health Canada must protect confidential business information in accordance with the Access to Information Act and the Food and Drugs Act. Currently Health Canada fees are. Applications and submissions for drug Health Details.
Master File MF Application Fee Form for Human Drugs 2020-03-23 in effect until March 31 2021 DOC Version - 58 KB Master File MF Application Fee Form for Human Drugs in effect April 1 2021 DOC Version - 55 KB Non-prescription drug monograph attestation form PDF fillablesaveable - 648 KB 2016-01-15. Note Fee Form. The revised fee structure increases the cost of filing a new DMF to 424 Canadian the cost of filing a biannual update to 191 Canadian and the cost of filing a Letters of Access to 191 Canadian.
Master File MF Application Fee Form for Human Drugs Note If you issue multiple LOAs referencing to the same DMF-No then the. Health Canada has increased Drug Master File DMF filing fees effective as of April 1 2015. New Master File Registration - 127300 Canadian Dollar CAD Update - 55200 CAD.
Fees for the right to sell medical devicesHealth Canada monitors medical devices on the Canadian market through post-market surveillance and compliance and enforcement activities. The DMF program serves as a reference to Health Canadas drug evaluators by providing confidential. Amount of payment will increase every year so please use current form httpswwwcanadacaenhealth-canadaservicesdrugs-health-productsdrug-productsapplications-submissionsformshtml.
Fees Paid by. For all other medical devices guidance documents and forms for. X 170 Cdn Total Fee sum of the above.
CustomerClient Account Number if issued. Master Files MFs - Procedures and Administrative Requirements 2017-04-28 Drug Master File Applications Form 2013-12-09 Master Files MF Application Fee Form for Human Drugs 2021-04-01. DMF Fees in Canada9 Drug Master FileDMF Fee Form Drug Master File Name.
Drug Master File DMF Application Form PDF fillablesaveable - 490 KB 2012-03-13 Microsoft Word version - 38 KB Drug Submission Application Fee Form for Human and Disinfectant Drugs 2020-03-23 in effect until March 31 2021 Microsoft Word. The new Master File fees are. X 520 Cdn Number of Letters of Access Enclosed.
Fees For Medical Devices. X 1200 Cdn MF Update.
What Is The Difference Between Gmp Fda Dmf And Cep Pharmaoffer
Timeline For Decision Making Framework Dmf Guide Development Process Download Scientific Diagram
Health Canada New Deadline For Dmf In Ectd Format
Pdf Regulatory Requirements For Drug Master File In Context To Canada And Australia
Capscanada A World Leading Manufacturer Of Hard Capsules Gelatin Capsules Capsule Empty Capsules
Pdf International Journal Of Drug Regulatory Affairs Filing Of Dmf In The Us Eu And India And Its Comparative Review
Pdf Dmf Filing In Us Europe And Canada
Https Www Schott Com D Pharmaceutical Packaging 3c1826ec 366b 4222 B9ee 78353e68966f 1 6 Ra Notice Loa To Agency Pdf
Pdf Dmf Filing Procedure In Us Europe And Canada A Review
Pdf New Advancements In Drug Master Files Guidelines
Rivaroxaban Api Dmfs Us Drug Master File Dmf Details Pharmacompass Com
Api Drug Master File All About Drugs
Pdf Dmf Filing Procedure In Us Europe And Canada A Review
Effects Of Delayed Release Dimethyl Fumarate Dmf On Health Related Quality Of Life In Patients With Relapsing Remitting Multiple Sclerosis An Integrated Analysis Of The Phase 3 Define And Confirm Studies Clinical Therapeutics
Agent Appointment Letter Template Example Lettering Letter Template Letter Templates
Active Pharmaceutical Ingredients Apis Jungbunzlauer
Pdf Regulatory Requirements For Drug Master File In Context To Canada And Australia



Posting Komentar untuk "Health Canada Dmf Fee Form"