Health Canada Medical Device License Application
The document requirements for each of the device class vary. The Medical Devices Bureau Bureau of the Therapeutic Products Directorate Health Canada is the Canadian federal regulator responsible for licensing medical devices in accordance with the Food and Drugs Act and Regulations and the Medical Devices Regulations.
Health Canada Application Forms For Obtaining Product Licences To Sell Natural Health Products In Canada Natural Health Health Application Form
Class II III and IV devices shall apply for a Canadian Medical Device License MDL application.

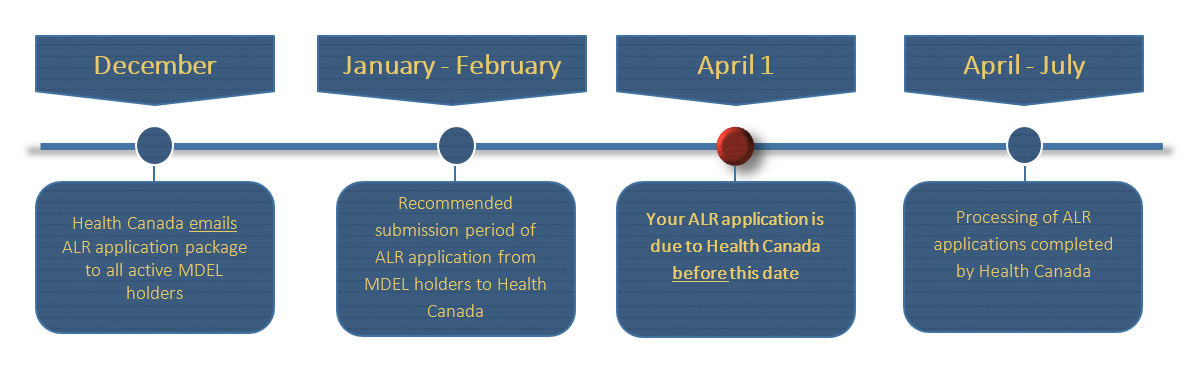
Health canada medical device license application. Health Canada MDEL Application CanSummit Canadian Medical Devices Market Consultants We are the Canadian Market Specialists Commercial Health Canada for the MedTech Medical Surgical Devices In-vitro Diagnostics IVD SaMD and BioTech sectors. Medical Device Establishment Licence MDEL application. Class II III and IV medical devices must be licenced before they may be imported or sold in Canada.
Emergo can also act your regulatory correspondent with Health Canada for your MDL. Class I devices can apply for Medical Device Establishment License MDEL by preparing mandatory procedures and paying Health Canada fees. Health Canadas performance targets were set at 15 days for first decisionsthat is decisions whether to issue MDLs or request additional informationfor Class II Medical Device License MDL applications 60 days for Class III MDL applications and 75 days for Class IV MDL applications.
From looking online and at the Health Canada website I found the following forms and guidances Class 3 non-in vitro diagnostic devices nIVD new and amendment applications Which breaks down the application content for Class III devices. A licence is issued to the device manufacturer for each application submitted provided the requirements of the Medical Devices Regulations are met. Medical Device License MDL.
New MDLs mostly on target. We manage the entire application process for Health Canada Medical Device License MDL for Class II III or IV medical or surgical devices IVD POCT-NPT SaMD. Device Licenses for Ultrasonic Diagnostic Systems and Transducers Health Canada the countrys regulating authority in the sphere of medical devices and other healthcare products has published a guidance document dedicated to medical device license applications for ultrasound diagnostic systems and transducers.
Class I represents the lowest risk and Class IV represents the highest risk. Class II devices require a distribution license from Health Canada. Medical devices are classified into one of 4 classes.
The guidance specifies scientific information requirements for manufacturers submitting Class III and Class IV device license applications excluding makers of Class III and IV IVD products. Fees for examination of application for an establishment license Medical Devices. For this you already need your ISO 134852003 certification from a CMDCAS registrar there are 14.
The review of Medical Device Licence Applications new licence the requests for the reinstatement of an existing licence as well as the amendment of a current medical device licence are subject to fees. This window is identical to the original MDALL search and displays the results as before. Completing and filing the Canadian Medical Device License MDL or Medical Device Establishment License MDEL application on your behalf.
Health Canada will increase fees regarding examination of application for each class of medical devices for the Fiscal Year 2020-2021. Medical Device Establishment License MDEL. The product is classified as Class III license type - medical device system.
Below is a detailed table of the fees. In order to market their devices in Canada manufacturers must obtain a license. Health Canada has released final guidance on supporting evidence requirements for Class III and Class IV medical device license applications.
Retrieved from the Final Report published by Health Canada. Selecting the Active Licence Search link takes you to the Medical Devices Active Licence Search window. Developing implementing or modifying your ISO 13485 quality management system to meet MDSAP and Canadian requirements.
The Licence Number query was improved to return the exact number match only. Instructions FRM-0292 2020-04-01 Medical Devices Licence Amendment Fax-Back Form - Guidance for Changes to the Manufacturers Name and or Address of Existing Device Licences Only 2020-04-21 PDF Version 130 KB DOC Version - 30 KB. Some of your devices may fall into this category and in those cases you would not be able to rely upon a distributors license and you would need to apply for your own.
Medical Report Form 5 Free Documents In Word Pdf With Medical Report Form Medical Report Medical Medical Examination
Toronto Nov 16 2017 Groundbreaking Medical Device Now Licensed With Health Canada Seqex Has Been Approved As A Class Ii Medi Medical Health Medical Device
Get Nurse Email List Registered Nurse Database From Medicoreach Nurse Registered Nurse Email List
There Are A Wide Range Of Medical Products From Thermometers To Hip Implants To Heart Valves And More That Qualify As Medical Devices Pharmaceutical Regul
Natural Health Product Licence Application Form User Manual Natural Health Application Form Homeopathic Medicine
Mevion 2 17 Photo 1 Highres Jpg 4 888 4 486픽셀 Medical Design Medical Device Design Treat Cancer
Ossix Volumax A New Ossifying Collagen Scaffold For Gbr And Gtr With Volume Maintaining Features Collagen Periodontitis Dental Implants
Icymi Health Canada Issued Ivwatch A Medical Device License Canada Is One Of The Most Progressive Health Care Markets In The W Medical Device Medical Health
43 Physical Exam Templates Forms Male Female Medical Examination Physics Medical
Usa Work Permit Documents Yahoo Search Results Image Search Results Canada Tourist British Visa Application Form
Consumer Labelling And Packing Regs Consumer Packaging Labels Consumers
Letter Of Invitation Canada How To Write One Sample Included Sample Of Invitation Letter Lettering Letter To Parents
Pin By Elsevier Health Us On Internal Medicine Medical Education Effective Teaching Medical Textbooks
Pin On Fda Update On Medical Device Cybersecurity
Pin On Portfolio Brochure Shape
Letter Of Invitation Canada How To Write One Sample Included Sample Of Invitation Letter Lettering Letter To Parents



Posting Komentar untuk "Health Canada Medical Device License Application"