Health Canada Medical Device Classification Database
From Health Canada A Medical Device Establishment Licence MDEL is a licence issued to Class I manufacturers as well as importers or distributors of all device classes to permit them to import or distribute a medical device in Canada. All of the data comes from Health Canada except for the categories Manufacturer Parent Company and Product Classification.
Sample Pico Questions Google Search Radiology Schools Nursing School School Work
The fdb prizm medical device database delivers knowledge for supply chain and clinical it systems to help improve clinical operational and financial.
Health canada medical device classification database. Medical Devices Active Licence Listing MDALL - Your reference tool for licensed medical devices in Canada. Health 4 days ago Health Canada holds the medical device manufacturers importers and distributors as accountable to correctly classify their medical devices as per Health Canada regulations. Classification of Medical Devices To determine the classification of a device you must apply all of the rules in Schedule 1 of the Medical Devices Regulations.
In Canada all Medical Devices are regulated by Health Canada Health Products and Food Branch Therapeutic Products Directorate Medical Devices Bureau. The purpose of the Medical Device Keyword Index is to assist manufacturers in verifying the classification of medical device products after application of the Classification Rules for Medical Devices set out in Schedule 1 of the Medical Devices Regulations. The Bureau maintains a database of all licensed Class II III and IV medical devices offered for sale in Canada.
Health canada will invoice fees over 5000. Class I medical devices do not require a medical device licence and are monitored by the Health Products and Food Branch Inspectorate Compliance and Enforcement through Establishment Licensing. What about a tissue.
The purpose of this document is to provide a comprehensive list of Health Canadas drug and medical device databases. These guidelines are designed to facilitate access to these databases and to inform stakeholders on what information is available. This includes any marketing material.
Canada with some of the stringent guidelines has one of the best Regulatory systems in the world for medical devices. B Examples of software that is not a medical device. The Canadian classification rules are located on pages 54-57 of the Canadian Medical Device Regulations httpbitlyFindCMDR.
These databases include information on prescription and non-prescription pharmaceuticals biologics and radiopharmaceuticals for use in humans and animals as well as medical devices. Selecting the Active Licence Search link takes you to the Medical Devices Active Licence Search window. Class I IIa IIb and III are the European classifications while Class I II III and IV are the Canadian classifications.
The FDA estimates 74 percent of Class I devices are exempt from premarket notifications. The Parent Company and the Product Classification were added by ICIJ. Only calibrators testers and quality control support devices offered for sale as part of medical device systems or as medical devices.
You must consider the labelled indications for use or claims made for the device. Health Canada also helps by giving some examples of software that it does not consider to be medical devices. Sometimes people can assume that the Health Canada.
Health Canada Medical Device Classification Service CanSummit Canadian Medical Devices Market Consultants We are the Canadian Market Specialists Commercial Health Canada for the MedTech Medical Surgical Devices In-vitro Diagnostics IVD SaMD and BioTech sectors. Only products which appear in this database listing may be offered for general marketing purposes in Canada. Health Canada Medical Device Classification Service.
Pure communication systems such as MDDS telephones etc. However some devices may have exemptions to certain requirements. The Medical Device Keyword Index should also be used in conjunction with the guidance document titled Guidance for the Risk-based Classification.
Classification levels are also risk based Class III as highest-risk for the patient or the administrator. Health Canada reviews Medical Devices to assess their safety effectiveness and quality before being authorized for sale in Canada. This window is identical to the original MDALL search and.
Dear visitor We have reorganized our Web site. However Health Canada does not classify all systems designed to help decision-making as a medical device. Canadian data is current through March 2018.
The first states that should the device act as a calibrator tester or quality control support to another medical device it is classified as Class II. All devices are subject to general controls. Classification of products is the first step in any regulatory review process at the Health Products and Food Branch HPFB.
When the classification of a health product is not evident the Office of Science of the Therapeutic Products Directorate is consulted. There are four European and Canadian medical device classifications. The parent company information is based on.
Software As Medical Device Samd Classification And Definitions
Blockchain For Financial Institutions Differences Vs Existing Architectures Statistics Chart Blockchain Technology Blockchain Financial Institutions
National Human Genome Research Institute Talking Glossary Of Genetic Terms Includes Pronunciation Definitions Illus Human Genome Genome Research Institute
12 The Dental Examination Dental Charting Dental Hygiene School Dental Assistant Study
Software Development To Scrape Company And Contact Information Web Scraping Expert Web Scraping Services B2b Email Lists Sales Leads Database Marketing Data Mining Data
What Is Samd Everything About Software As A Medical Device
Medical Device Regulations Classification Submissions
How To Overcome The Flaw In Hotel Revenue Management Sponsored Hotel Revenue Management Revenue Management Hotel Management
Class Ii Iv Medical Device Investigational Testing In Canada Vantage Biotrials
Clinical Trials Medical Device Trials Genesis Research Services
Software As Medical Device Samd Classification And Definitions
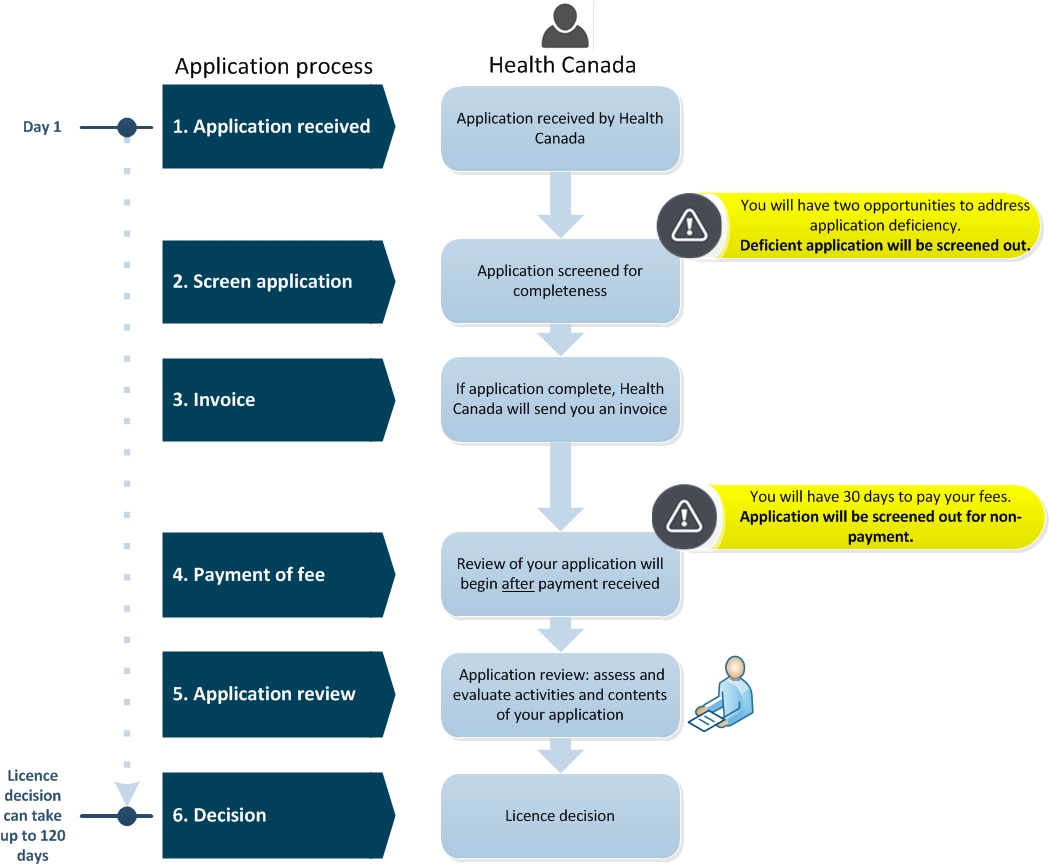
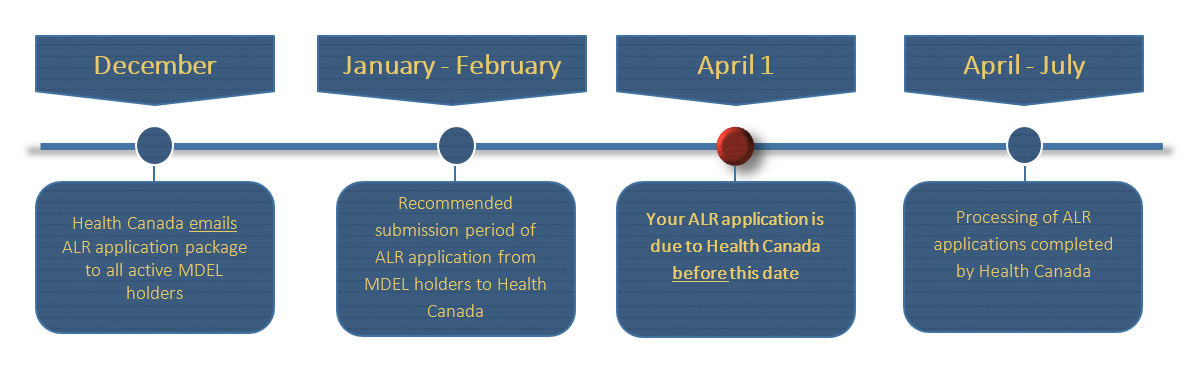
Frequently Asked Questions Medical Device Establishment Licensing And Fees Canada Ca
Best Photos Of Human Resources Incident Report Template Within First Aid Incident Report Form Template C Incident Report Form Incident Report Report Template
Click On Image To Zoom Medical Research Scientific Publication Cohort Study
Clinical Trials Medical Device Trials Genesis Research Services



Posting Komentar untuk "Health Canada Medical Device Classification Database"